Artículos SCI
2021
2021
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Examination of the Deactivation Cycle of NiAl- and NiMgAl-Hydrotalcite Derived Catalysts in the Dry Reforming of Methane
Abdelsadek, Z.; Holgado, J.P.; Halliche, D.; Caballero, A.; Cherifi, O.; Gonzalez-Cortes, S.; Masset, P.J.Catalysis Letters, 151 (2021) 2696-2715
Show abstract ▽
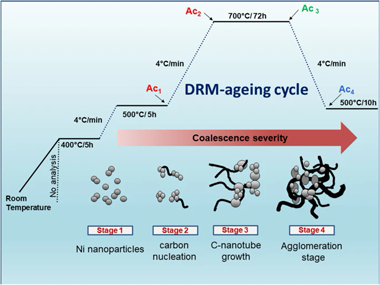
The importance of the dry reforming of methane (DRM) lies in its capability to upgrade two greenhouse gases (CH4 and CO2) into synthesis gas (CO and H-2), which is one of the main building block for synthesizing hydrocarbons. However, the Ni-based catalysts for DRM reaction usually have a major catalytic stability drawback. This works aims to assess the catalytic activity and stability of two Ni-based catalysts obtained from hydrotalcite (HT) precursors (i.e., NiAl-HT and NiMgAl-HT). The precursors, calcined (-c), reduced (-R) and spent samples were characterized by a series of techniques to gain insight into the influence of MgO over Ni-based catalyst in the drying reforming of methane. An in-situ ageing cycle process to speed up the deactivation of hydrotalcite-derived catalysts showed that the NiMgAl-HTc-R catalyst displayed a higher activity and resistance to coke formation (stability) than NiAl-HTc-R because of the introduction of Mg into hydrotalcite structure in the catalyst precursor. The presence of this element enhances several factors involved in the stability of Ni-based catalysts for the DRM process such as the reducibility and textural features of the catalysts, size and dispersion of Ni-0 nanoparticles and also maintains a good compromise between the acid and base properties of the solid catalysts.
Septiembre, 2021 | DOI: 10.1007/s10562-020-03513-4
Materiales Nanoestructurados y Microestructura
Pd-C Catalytic Thin Films Prepared by Magnetron Sputtering for the Decomposition of Formic Acid
Arzac, GM; Fernandez, A; Godinho, V; Hufschmidt, D; de Haro, MCJ; Medran, B; Montes, ONanomaterials, 11 (2021) 2326
Show abstract ▽
Formic acid is an advantageous liquid organic hydrogen carrier. It is relatively nontoxic and can be synthesized by the reaction of CO2 with sustainable hydrogen or by biomass decomposition. As an alternative to more widely studied powdery catalysts, supported Pd-C catalytic thin films with controlled nanostructure and compositions were newly prepared in this work by magnetron sputtering on structured supports and tested for the formic acid decomposition reaction. A two-magnetron configuration (carbon and tailored Pd-C targets) was used to achieve a reduction in Pd consumption and high catalyst surface roughness and dispersion by increasing the carbon content. Activity and durability tests were carried out for the gas phase formic acid decomposition reaction on SiC foam monoliths coated with the Pd-C films and the effects of column width, surface roughness and thermal pre-reduction time were investigated. Activity of 5.04 mol(H2)center dot g(Pd)(-1)center dot h(-1) and 92% selectivity to the dehydrogenation reaction were achieved at 300 degrees C for the catalyst with a lower column width and higher carbon content and surface roughness. It was also found that deactivation occurs when Pd is sintered due to the elimination of carbon and/or the segregation and agglomeration of Pd upon cycling. Magnetron sputtering deposition appears as a promising and scalable route for the one-step preparation of Pd-C catalytic films by overcoming the different deposition characteristics of Pd and C with an appropriate experimental design.
Septiembre, 2021 | DOI: 10.3390/nano11092326
Química de Superficies y Catálisis
Structure-sensitivity of formic acid dehydrogenation reaction over additive-free Pd NPs supported on activated carbon
Santos, J.L.; Megías-Sayago, C.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A.Chemical Engineering Journal, 420 (2021) 127641
Show abstract ▽
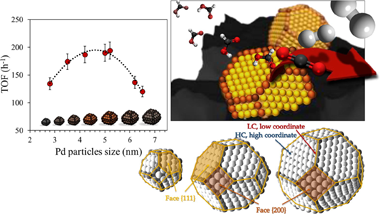
In this study the size-activity dependence of palladium based catalysts in formic acid dehydrogenation reaction was investigated and evaluated. A wide range of particle sizes was considered and the catalyst series were prepared upon variation of some synthetic parameters, precursor and solvent nature in particular. Synthesis method variations affect significantly Pd particle size and results in diverse activity toward hydrogen production. An optimal size was observed and explained by the diverse proportion of low and high coordinated Pd states available for different samples within the series. The evaluation of particles much bigger than 6 nm changes importantly the fraction of high and low coordination atoms and allows the clear confirmation of the importance of the presence of low coordination atoms on the surface of catalyst.
Septiembre, 2021 | DOI: 10.1016/j.cej.2020.127641
Química de Superficies y Catálisis
Mesoporous Carbon Production by Nanocasting Technique Using Boehmite as a Template
Ortega-Franqueza, M; Ivanova, S; Dominguez, MI; Centeno, MACatalysts, 11 (2021) 1132
Show abstract ▽
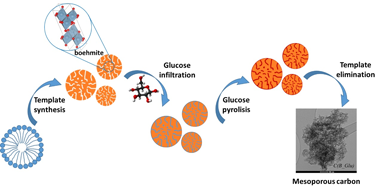
A series of mesoporous carbonaceous materials were synthesized by the nanocasting technique using boehmite as a template and glucose as a carbon precursor. After pyrolysis and template removal, the resulting material is a mesoporous carbon that can be additionally doped with N, B and K during prepyrolysis impregnation. In addition, the influence of doping on the morphology, crystallinity and stability of the synthesized carbons was studied using X-ray diffraction, nitrogen physisorption, thermogravimetry, Raman and IR spectroscopy and transmission electron microscopy. While the nanocasting process is effective for the formation of mesopores, KOH and urea do not modify the textural properties of carbon. The use of H3PO4 as a dopant, however, led to the formation of an AlPO4 compound and resulted in a solid with a lower specific surface area and higher microporosity. All doped solids present higher thermal stability as a positive effect of the introduction of heteroatoms to the carbon skeleton. The phosphorus-doped sample has better oxidation resistance, with a combustion temperature 120-150 degrees C higher than those observed for the other materials.
Septiembre, 2021 | DOI: 10.3390/catal11091132
Reactividad de Sólidos
Calcination under low CO2 pressure enhances the calcium Looping performance of limestone for thermochemical energy storage
Sarrion, B; Perejon, A; Sanchez-Jimenez, PE; Amghar, N; Chacartegui, R; Valverde, JM; Perez-Maqueda, LAChemical Engineering Journal, 417 (2021) 127922
Show abstract ▽
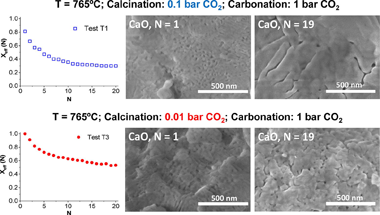
The Calcium Looping performance of limestone for thermochemical energy storage has been investigated under novel favorable conditions, which involve calcination at moderate temperatures under CO2 at low pressure (0.01 and 0.1 bar) and carbonation at high temperature under CO2 at atmospheric pressure. Calcining at low CO2 pressures allows to substantially reduce the temperature to achieve full calcination in short residence times. Moreover, it notably enhances CaO multicycle conversion. The highest values of conversion are obtained for limestone samples calcined under 0.01 bar CO2 at 765 degrees C. Under these conditions, the residual conversion is increased by a factor of 10 as compared to conditions involving calcination under CO2 at atmospheric pressure. The enhancement of CaO conversion is correlated to the microstructure of the CaO samples obtained after calcination. As seen from SEM, BET surface and XRD analysis, calcination under low CO2 pressure leads to a remarkable decrease of pore volume and CaO crystallite size. Consequently, CaO surface area available for carbonation in the fast reaction-controlled regime and therefore reactivity in short residence times is promoted.
Agosto, 2021 | DOI: 10.1016/j.cej.2020.127922
- ‹ anterior
- 69 of 410
- siguiente ›














