Artículos SCI
2020
2020
Química de Superficies y Catálisis
Metal catalysts supported on biochars: Part I synthesis and characterization
Santos, JL; Maki-Arvela, P; Monzon, A; Murzin, DY; Centeno, MAApplied Catalysis B-Environmental, 268 (2020) 118423
Show abstract ▽
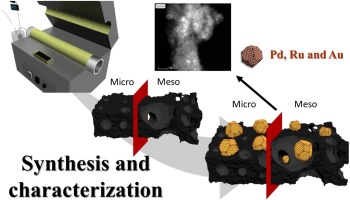
In the current study, synthesis and detailed characterization of cellulose biochars as a waste biomass model component and vine shoot biochars as a real waste biomass catalyst was performed. Although initially biochars exhibit poor textural properties, a simple activation process can make them much more suitable as a catalyst supports. A combination of physical (CO2) and chemical activation (ZnCl2) was evaluated. The characterization results indicated that the surface area and pore volume of the biochars have increased significantly by chemical activation treatment with ZnCl2. A series of metal catalysts (Pd, Au and Ru) supported on biochars was prepared and characterized. The prepared materials represent a set of noble metal catalysts supported on biochars with different textural and surface properties, which can be used to evaluate the catalytic role of the active phase and carbon support nature in catalytic reactions of interest, such as hydrodeoxygenation, described in the part II.
Julio, 2020 | DOI: 10.1016/j.apcatb.2019.118423
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
(NH4)4[NiMo6O24H6].5H2O / g-C3N4 materials for selective photo-oxidation of Csingle bondO and Cdouble bondC bonds
Caudillo-Flores, U; Ansari, F; Bachiller-Baeza, B; Colon, G; Fernandez-Garcia, M; Kubacka, AApplied Catalysis B-Environmental, 278 (2020) 119299
Show abstract ▽
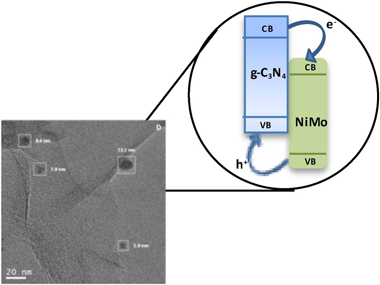
Novel composite photo-catalysts having (NH4)(4)[NiMo6O24H6]center dot 5H(2)O Polyoxometalate (POM) species deposited over g-C3N4 are synthesized. Materials were characterized through a multitechnique approach showing the stability of the carbon nitride component both through the synthesis process and under reaction. Contrarily, the POM component evolves under reaction conditions to maximize the interaction with the support. Such a behavior renders, as measured by the quantum efficiency, highly active photo-catalysts in the photo-oxidation of 2-propanol and styrene both under UV and sunlight illumination, setting up the basis for a green catalytic process. The material having a 4 wt. % POM showed improved activity with respect to both parent constituents but also higher selectivity to the partial oxidation of the alcohol and the aromatic hydrocarbon to generate added value chemical compounds. A multitechnique approach investigating charge carrier fate demonstrates the key role played by the interaction between components to promote activity and selectivity in selective oxidation reactions.
Diciembre, 2020 | DOI: 10.1016/j.apcatb.2020.119299
Química de Superficies y Catálisis
Hydrodeoxygenation of vanillin over noble metal catalyst supported on biochars: Part II: Catalytic behaviour
Santos, JL; Maki-Arvela, P; Warna, J; Monzon, A; Centeno, MA; Murzin, DYApplied Catalysis B-Environmental, 268 (2020) 118425
Show abstract ▽
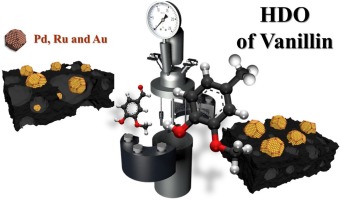
Vanillin hydrodeoxygenation was investigated using noble metal (Pd, Au, Ru) supported on active carbon prepared from waste derived biochars, which were produced via pyrolysis in CO2 atmosphere. Chemical activation with ZnCl2 and HNO3 was also used in the preparation of active carbon to enhance the specific surface area and demineralize material, respectively. Both fresh and spent catalysts were characterized with X-ray diffraction, DRIFTS, zeta potential measurement and HR-TEM. The highest selectivity to p-creosol, 92 % selectivity at complete vanillin conversion after 3 h was obtained in vanillin hydrodeoxygenation at 100 degrees C under 30 bar in hydrogen in water with Pd/C catalyst prepared via pyrolysis under CO2 from wine waste and using ZnCl2 as a chemical activation agent. Hydrodeoxygenation activity increased with increasing metal dispersion. A kinetic model including adsorption of vanillin described well the experimental data.
Julio, 2020 | DOI: 10.1016/j.apcatb.2019.118425
Química de Superficies y Catálisis
Experimental evidence of HCO species as intermediate in the fischer tropsch reaction using operando techniques
Diaz-Sanchez, RM; de-Paz-Carrion, A; Serrera-Figallo, MA; Torres-Lagares, D; Barranco, A; Leon-Ramos, JR; Gutierrez-Perez, JLApplied Catalysis B-Environmental, 272 (2020) 119032
Show abstract ▽
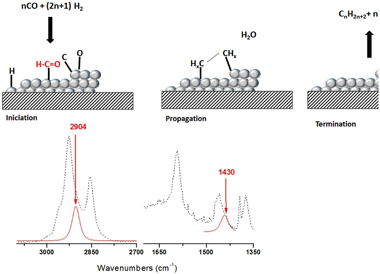
Fischer Tropsch's reaction, known from 1925, receives special attention nowadays due to its key role in the CO2 or biomass valorization to liquid fuels and chemicals. Several aspects on the exact mechanism or the role of water in this reaction are not yet completely clear. Formyl species, HCO, have been proposed as the most probable reaction intermediate, but they have never been observed under operation conditions closed to the real ones. In this work, using DRIFTS-MS operando techniques, HCO intermediates are detected under a H2/CO flow and 200 °C. IR bands at 2900 cm−1 and 1440 cm−1 attributed to ν(C–H) and δ(HCO) vibrations modes characterize these species. Evolution of these bands with the reaction time evidences its high reactivity with OH groups, which explains the positive effect of water on the CO conversion previously observed.
Septiembre, 2020 | DOI: 10.1016/j.apcatb.2020.119032
Nanotecnología en Superficies y Plasma
Chemistry and Electrocatalytic Activity of Nanostructured Nickel Electrodes for Water Electrolysis
Lopez-Fernandez, E; Gil-Rostra, J; Espinos, JP; Gonzalez-Elipe, AR; Consuegra, AD; Yubero, FACS Catalysis, 10 (2020) 6159-6170
Show abstract ▽
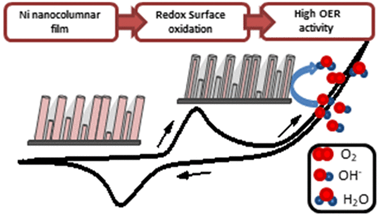
Herein we have developed nanostructured nickel-based electrode films for anion exchange membrane water electrolysis (AEMWE). The electrodes were prepared by magnetron sputtering (MS) in an oblique angle configuration and under various conditions aimed at preparing metallic, oxide, or oxyhydroxide films. Their electrochemical analysis has been complemented with a thorough physicochemical characterization to determine the effect of microstructure, chemical state, bilayer structure, and film thickness on the oxygen evolution reaction (OER). The maximum electrocatalytic activity was found for the metallic electrode, where analysis by X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS) demonstrated that the active catalytic phase at the surface after its electrochemical conditioning is a kind of oxidized nickel oxide/hydroxide layer with the thickness of a few nanometers. Electrochemical impedance spectroscopy analysis of these steady-state working electrodes supports that the enhanced performance of the metallic nickel anode vs other chemical states resides in the easier electron transfer through the electrode films and the various interlayers built up during their fabrication and activation. The long-term steady-state operation of the anodes and their efficiency for water splitting was proved in a full-cell AEMWE setup incorporating magnetron-sputtered metallic nickel as the cathode. This work proves that MS is a suitable technique to prepare active, stable, and low-cost electrodes for AEMWE and the capacity of this technique to control the chemical state of the electrocatalytically active layers involved in the OER.
Junio, 2020 | DOI: 10.1021/acscatal.0c00856
- ‹ anterior
- 89 of 410
- siguiente ›
icms











