Artículos SCI
2021
2021
Materiales Ópticos Multifuncionales - Materiales Coloidales
Persistent luminescent nanoparticles: Challenges and opportunities for a shimmering future
Castaing, V.; Arroyo, E.; Becerro, A.I.; Ocaña, M.; Lozano, G.; Míguez, H.Journal of Applied Physics, 130 (2021) 080902
Show abstract ▽
Persistent phosphors are luminescent sources based on crystalline materials doped with rare-earth or transition metal cations able to produce light after the excitation source vanishes. Although known for centuries, these materials gained renewed interest after the discovery of Eu2+,RE3+ co-doped aluminates and silicates in the late 1990s due to their unprecedented afterglow properties. In contrast, persistent nanophosphors have emerged only recently as a nanoscale alternative to their bulk counterparts, offering exciting opportunities of particular relevance for in vivo imaging, optical data storage, or unconventional light generation. However, taking advantage of the avenues opened by nanoscience demands developing new synthetic strategies that allow precise control of the morphology, surface, and defect chemistry of the nanomaterials, along with a profound understanding of the physical mechanisms occurring in the nanoscale. Besides, advanced physicochemical characterization is required to assess persistent luminescence in a quantitative manner, which allows strict comparison among different persistent nanophosphors, aiming to propel their applicability. Herein, we revisit the main phenomena that determine the emission properties of persistent nanoparticles, discuss the most promising preparation and characterization protocols, highlight recent achievements, and elaborate on the challenges ahead.
Agosto, 2021 | DOI: 10.1063/5.0053283
Reactividad de Sólidos
Kinetics and cyclability of limestone (CaCO3) in presence of steam during calcination in the CaL scheme for thermochemical energy storage
Arcenegui-Troya, J; Sanchez-Jimenez, PE; Perejon, A; Moreno, V; Valverde, JM; Perez-Maqueda, LAChemical Engineering Journal, 417 (2021) 129194
Show abstract ▽
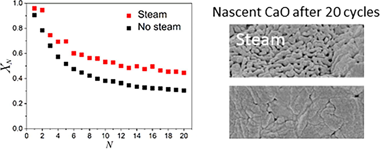
In the present work, we explore the use of steam in the CaCO3 calcination step of the Calcium Looping process devised for thermochemical energy storage (CaL-TCES). Steam produces a double benefit: firstly, it fastens calcination, allowing a reduction of the temperature needed to attain full calcination in short residence times, as those required in practice, resulting in energy savings. This behaviour is justified on the basis of a kinetics study results obtained from a non-parametric kinetic analysis, which demonstrate that the presence of steam during calcination can reduce the apparent activation energy from 175 kJ/mol to 142 kJ/mol with a steam's partial pressure of 29%. In addition, the results obtained for multicycle CaL-TCES tests show that steam alleviates the deactivation of the sorbent, which is one of the main limiting factors of this technology. This behaviour is explained in terms of the effect of steam on the microstructure of the regenerated CaO. Importantly, the values of residual conversion attained by calcining in steam are higher than those without steam.
Agosto, 2021 | DOI: 10.1016/j.cej.2021.129194
Reactividad de Sólidos
Study of the Influence of Sintering Atmosphere and Mechanical Activation on the Synthesis of Bulk Ti2AlN MAX Phase Obtained by Spark Plasma Sintering
Salvo, C; Chicardi, E; García-Garrido, C; Poyato, R; Jimenez, JA; Mangalaraja, RVMaterials, 14 (2021) 4574
Show abstract ▽
The influence of the mechanical activation process and sintering atmosphere on the microstructure and mechanical properties of bulk Ti2AlN has been investigated. The mixture of Ti and AlN powders was prepared in a 1:2 molar ratio, and a part of this powder mixture was subjected to a mechanical activation process under an argon atmosphere for 10 h using agate jars and balls as milling media. Then, the sintering and production of the Ti2AlN MAX phase were carried out by Spark Plasma Sintering under 30 MPa with vacuum or nitrogen atmospheres and at 1200 degrees C for 10 min. The crystal structure and microstructure of consolidated samples were characterized by X-ray Diffraction, Scanning Electron Microscopy, and Energy Dispersive X-ray Spectroscopy. The X-ray diffraction patterns were fitted using the Rietveld refinement for phase quantification and determined their most critical microstructural parameters. It was determined that by using nitrogen as a sintering atmosphere, Ti4AlN3 MAX phase and TiN were increased at the expense of the Ti2AlN. In the samples prepared from the activated powders, secondary phases like Ti5Si3 and Al2O3 were formed. However, the higher densification level presented in the sample produced by using both nitrogen atmosphere and MAP powder mixture is remarkable. Moreover, the high-purity Ti2AlN zone of the MAX-1200 presented a hardness of 4.3 GPa, and the rest of the samples exhibited slightly smaller hardness values (4.1, 4.0, and 4.2 GPa, respectively) which are matched with the higher porosity observed on the SEM images.
Agosto, 2021 | DOI: 10.3390/ma14164574
Materiales Avanzados
Geopolymers made from metakaolin sources, partially replaced by Spanish clays and biomass bottom ash
Eliche-Quesada, D; Calero-Rodriguez, A; Bonet-Martinez, E;Perez-Villarejo, L; Sanchez-Soto, PJJournal of Building Engineering, 40 (2021) 102761
Show abstract ▽
The main objective of this investigation is to study the effect of the substitution of metakaolin (MK) (from calcined industrial kaolin) by four different calcined natural Southern Spain clays traditionally used in the brick and tile sector, as well as by the biomass bottom ash residue (BBA) from the combustion of a mix of olive and pine pruning on the synthesis of geopolymer with physical, mechanical and thermal properties comparable to those of classic construction materials. As alkaline activator, a 8 M solution of sodium hydroxide and sodium silicate have been used. Raw materials, metakaolin; Spanish clays: black clay (BC), yellow clay (YC), white clay (WC), red clay (RC) and BBA were characterized by chemical analysis (XRF), mineralogical analysis (XRD), and particle size analysis. Control geopolymers containing only metakaolin, and batch of geopolymers were formulated containing equal proportions of metakaolin, BBA and each of the four types of clay. After the curing period, at 60 degrees C for 1 day geopolymers were demolded and stored 27 days at room temperature. Geopolymers were characterized using Scanning Electron Microscopy coupled with Energy Dispersive Spectroscopy (SEM-EDS), XRD and Attenuated Total Reflectance-Fourier Transform Infrared Spec troscopy (ATR-FTIR). Their physical, mechanical and thermal properties have also been studied. The addition of BBA and different types of calcined clays to metakaolin gives rise to geopolymers with higher mechanical properties increasing the compressive strength of the control geopolymer containing only MK (24.9 MPa) by more than 50% for the GMK-BBA-WC geopolymers (38.5 MP a). The clays act as fillers and/or promote the precipitation of calcium-rich phases (Ca)-A-S-H-G gel that coexists with the (Na)-A-S-H gel type. The relevant results of physical, mechanical and thermal properties obtained in this research demonstrate the potential of Spanish clays and BBA as binders and substitutes for metakaolin.
Agosto, 2021 | DOI: 10.1016/j.jobe.2021.102761
Reactividad de Sólidos
Calcination under low CO2 pressure enhances the calcium Looping performance of limestone for thermochemical energy storage
Sarrion, B; Perejon, A; Sanchez-Jimenez, PE; Amghar, N; Chacartegui, R; Valverde, JM; Perez-Maqueda, LAChemical Engineering Journal, 417 (2021) 127922
Show abstract ▽
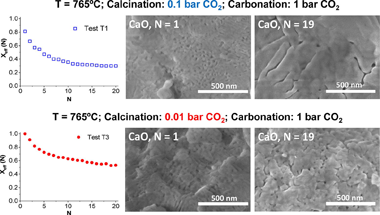
The Calcium Looping performance of limestone for thermochemical energy storage has been investigated under novel favorable conditions, which involve calcination at moderate temperatures under CO2 at low pressure (0.01 and 0.1 bar) and carbonation at high temperature under CO2 at atmospheric pressure. Calcining at low CO2 pressures allows to substantially reduce the temperature to achieve full calcination in short residence times. Moreover, it notably enhances CaO multicycle conversion. The highest values of conversion are obtained for limestone samples calcined under 0.01 bar CO2 at 765 degrees C. Under these conditions, the residual conversion is increased by a factor of 10 as compared to conditions involving calcination under CO2 at atmospheric pressure. The enhancement of CaO conversion is correlated to the microstructure of the CaO samples obtained after calcination. As seen from SEM, BET surface and XRD analysis, calcination under low CO2 pressure leads to a remarkable decrease of pore volume and CaO crystallite size. Consequently, CaO surface area available for carbonation in the fast reaction-controlled regime and therefore reactivity in short residence times is promoted.
Agosto, 2021 | DOI: 10.1016/j.cej.2020.127922
- ‹ anterior
- 72 of 412
- siguiente ›














