Artículos SCI
2021
2021
Materiales Avanzados
Geopolymers made from metakaolin sources, partially replaced by Spanish clays and biomass bottom ash
Eliche-Quesada, D; Calero-Rodriguez, A; Bonet-Martinez, E;Perez-Villarejo, L; Sanchez-Soto, PJJournal of Building Engineering, 40 (2021) 102761
Show abstract ▽
The main objective of this investigation is to study the effect of the substitution of metakaolin (MK) (from calcined industrial kaolin) by four different calcined natural Southern Spain clays traditionally used in the brick and tile sector, as well as by the biomass bottom ash residue (BBA) from the combustion of a mix of olive and pine pruning on the synthesis of geopolymer with physical, mechanical and thermal properties comparable to those of classic construction materials. As alkaline activator, a 8 M solution of sodium hydroxide and sodium silicate have been used. Raw materials, metakaolin; Spanish clays: black clay (BC), yellow clay (YC), white clay (WC), red clay (RC) and BBA were characterized by chemical analysis (XRF), mineralogical analysis (XRD), and particle size analysis. Control geopolymers containing only metakaolin, and batch of geopolymers were formulated containing equal proportions of metakaolin, BBA and each of the four types of clay. After the curing period, at 60 degrees C for 1 day geopolymers were demolded and stored 27 days at room temperature. Geopolymers were characterized using Scanning Electron Microscopy coupled with Energy Dispersive Spectroscopy (SEM-EDS), XRD and Attenuated Total Reflectance-Fourier Transform Infrared Spec troscopy (ATR-FTIR). Their physical, mechanical and thermal properties have also been studied. The addition of BBA and different types of calcined clays to metakaolin gives rise to geopolymers with higher mechanical properties increasing the compressive strength of the control geopolymer containing only MK (24.9 MPa) by more than 50% for the GMK-BBA-WC geopolymers (38.5 MP a). The clays act as fillers and/or promote the precipitation of calcium-rich phases (Ca)-A-S-H-G gel that coexists with the (Na)-A-S-H gel type. The relevant results of physical, mechanical and thermal properties obtained in this research demonstrate the potential of Spanish clays and BBA as binders and substitutes for metakaolin.
Agosto, 2021 | DOI: 10.1016/j.jobe.2021.102761
Reactividad de Sólidos
Calcination under low CO2 pressure enhances the calcium Looping performance of limestone for thermochemical energy storage
Sarrion, B; Perejon, A; Sanchez-Jimenez, PE; Amghar, N; Chacartegui, R; Valverde, JM; Perez-Maqueda, LAChemical Engineering Journal, 417 (2021) 127922
Show abstract ▽
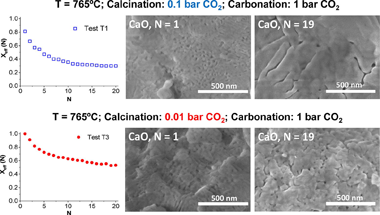
The Calcium Looping performance of limestone for thermochemical energy storage has been investigated under novel favorable conditions, which involve calcination at moderate temperatures under CO2 at low pressure (0.01 and 0.1 bar) and carbonation at high temperature under CO2 at atmospheric pressure. Calcining at low CO2 pressures allows to substantially reduce the temperature to achieve full calcination in short residence times. Moreover, it notably enhances CaO multicycle conversion. The highest values of conversion are obtained for limestone samples calcined under 0.01 bar CO2 at 765 degrees C. Under these conditions, the residual conversion is increased by a factor of 10 as compared to conditions involving calcination under CO2 at atmospheric pressure. The enhancement of CaO conversion is correlated to the microstructure of the CaO samples obtained after calcination. As seen from SEM, BET surface and XRD analysis, calcination under low CO2 pressure leads to a remarkable decrease of pore volume and CaO crystallite size. Consequently, CaO surface area available for carbonation in the fast reaction-controlled regime and therefore reactivity in short residence times is promoted.
Agosto, 2021 | DOI: 10.1016/j.cej.2020.127922
Reactividad de Sólidos
Mechanochemical synthesis of ternary chalcogenide chalcostibite CuSbS2 and its characterization
Dutkova, E; Sayagues, MJ; Fabian, M; Kovac, J; Kovat, J; Balaz, M; Stahorsky, MJournal of Materials Science-Materials in Electronics (2021)
Show abstract ▽
In this work, the very rapid one-step mechanochemical synthesis of nanocrystalline ternary chalcogenide chalcostibite CuSbS2 prepared from copper, antimony, and sulfur precursors by high-energy milling for only 30 min in a planetary mill is reported. XRD confirmed the orthorhombic crystal structure of CuSbS2. The crystallite size of CuSbS2 calculated by LeBail refinement of the X-ray powder diffraction data was 25 nm. The nanocrystalline chalcostibite CuSbS2 was also confirmed by transmission electron microscopy. The purity of CuSbS2 was verified by Raman spectroscopy. The synthesized chalcostibite exhibits the specific surface area value of 2.4 m(2)g(-1). UV-Vis spectroscopy showed the optical bandgap of CuSbS2 as 1.54 eV with wide range of absorption in visible region. Photoresponse of CuSbS2 was confirmed by I-V measurements under dark and light illumination. The proposed mechanochemical synthesis provides an alternative approach to prepare also other ternary semiconductor nanomaterials. CuSbS2 semiconductor nanocrystals have the potential to be used as light absorbers in photovoltaics.
Agosto, 2021 | DOI: 10.1007/s10854-021-06767-9
Reactividad de Sólidos
Tuning the excitation wavelength of luminescent Mn2+-doped ZnSxSe1-x obtained by mechanically induced self-sustaining reaction
Aviles, MA; Gotor, FJOptical Materials, 117 (2021) 111121
Show abstract ▽
Mn2+-doped ZnSxSe1-x solid solution samples (Mn:ZnSxSe1-x) were synthesized by the mechanochemical process denoted as mechanically-induced self-sustaining reaction from Mn/Zn/S/Se powder elemental mixtures. The samples were characterized by X-ray diffraction, scanning electron microscopy, diffuse reflectance UV-Vis spectroscopy and emission and excitation photoluminescence measurements. The band-gap energy of samples was controlled by changing the stoichiometry, x, of the solid solution. All samples showed the characteristic Mn2+ 4T1-6A1 emission at -588 nm when exciting the host material, so it was possible to tune the excitation wavelength from 349 nm to 467 nm. However, an efficiency loss was observed with increasing Se content, probably due to the overlap between the absorption and emission spectra that induced self-absorption and emission quenching.
Julio, 2021 | DOI: 10.1016/j.optmat.2021.111121
Archeometric characterization (physical-chemical and microstructural) of tiles in the Mudejar Palace of the Royal Alcazar of Seville using non-invasive quantitative chemical methods
Perez-Rodriguez, JL; Robador, MD; Castaing, J; de Viguerie, L; Garrote, MA; Pleguezuelo, ABoletin de la Sociedad Española de Ceramica y Vidrio, 60 (2021) 211-228
Show abstract ▽
The Palaces in the Alcazar of Seville, Spain, are famous for their ceramic decoration. The technique of tessellation was used extensively in all rooms in the Mudejar Palace, dated in the fourteenth century. These glazed ceramics have been analysed in situ using noninvasive quantitative chemical methods of X-ray fluorescence and diffraction (XRF and XRD). Micro-samples were taken to prepare cross-sections and analysed by optical and electronic microscopy. The composition of these ceramics, their manufacturing technique and the time of application in the different areas of the Palace have been characterized in this work. Five colours have been found in the glazed ceramics: green, black, molasses, white and blue. Fe, Co, Cu, Mn and Sn are the main chemical elements responsible for the colour of the glass phase of these ceramics. Wollastonite, quartz, bustamite and feldspars inclusions have been found in the glass phase. Casiterite and Malayaite have been also characterized by XRD. The ceramic paste used for manufacturing was calcic and was heated at about 900 degrees C. Thenardite, gypsum, sodium chloride and nitrogen compounds have been characterized in the ceramic and are responsible for their alteration. The information obtained in the 24 zones studied shows that there is no homogeneity in the ceramics due to the different times in which the tiles were placed and the restorations carried out over time. There are 3 main groups of ceramics: a) probably from 14th century, b), probably from 15-16th centuries and c) from 19-20th centuries and recent restorations.
Julio, 2021 | DOI: 10.1016/j.bsecv.2020.03.001
- ‹ anterior
- 71 of 410
- siguiente ›














