Artículos SCI
2022
2022
Química de Superficies y Catálisis
Understanding the promotional effect of Pt/CeO2 in cobalt-catalyzed Fischer-Tropsch synthesis using operando infrared spectroscopy at moderated pressures
Bobadilla, LF; Egana, A; Castillo, R.; Romero-Sarria, F.; Centeno, M.A.; Sanz, O.; Montes, M.; Odriozola, J.A.FUEL, 312 (2022) 122964
Show abstract ▽
Fischer-Tropsch (FTS) reaction is a well-known catalytic process for the conversion of synthesis gas into liquid fuels. The addition of a water gas shift (WGS) catalyst to the FTS one has been postulate to notably increase the efficiency of the process. In order to investigate this issue, we conducted the FTS reaction over a Co-Re/Al2O3 catalyst combined with an optimal WGS Pt/CeO2 catalyst. We observed a notable increase of CO conversion in presence of the Pt/CeO2 catalyst that a priori could be attributed to the WGS reaction. However, the WGS reaction is unfavourable at pressures higher than 1 bar and CO/CO2 hydrogenation over Pt/CeO2 could be more favoured under FTS reaction conditions. In order to gain insights on this fact and elucidate the role of Pt/CeO2 in the FTS reaction we have performed an operando DRIFTS-MS study under close FTS reaction conditions at 4 bar over the Pt/CeO2 catalyst.
Marzo, 2022 | DOI: 10.1016/j.fuel.2021.122964
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Preferential CO oxidation in hydrogen-rich gases over Ag catalysts supported on different supports
Todorova, S; Kolev, H; Karakirova, Y; Filkova, D; Grahovski, B; Aleksieva, K; Holgado, JP; Kadinov, G; Caballero, AReaction Kinetics Mechanisms and Catalysis, 135 (2022) 1405-1422
Show abstract ▽
The monometallic silver supported on SiO2, Al2O3, ZSM-5 (Si:Al = 100) and bi-metallic AgCe/SiO2 samples were studied in the reaction of the preferential CO oxidation. It was established that the supported silver catalysts are promising systems for selective oxidation of CO at low temperatures and the addition of cerium oxide increases the catalytic activity and selectivity most probably because of the increase in the silver dispersion; the homogeneous distribution of Ag and ceria on the silica support; formation of Ag-n(delta+) clusters; increase in bulk and subsurface oxygen.
Marzo, 2022 | DOI: 10.1007/s11144-022-02158-1
Reactividad de Sólidos
Predictions of polymer thermal degradation: relevance of selecting the proper kinetic model
Sanchez-Jimenez, PE; Perejon, A; Arcenegui-Troya, J; Perez-Maqueda, LAJournal of Thermal Analysis and Calorimetry, 147 (2022) 2335-2341
Show abstract ▽
Making predictions, such as lifetime estimations, is one of the main objectives of kinetic studies. Thus, from conventional thermal analysis experiments, the behavior of polymeric materials under processing or application conditions, usually far away from those used in the laboratory experiments, could be estimated. Conventional prediction procedures usually make use of oversimplified equations based on simple approaches. One of the most common approaches is the assumption of a first, or n-order, kinetic model for the process. However, recent studies have shown, for a number of polymers, that random scission kinetic models are not only physically sound, but more reliable in terms of describing the degradation kinetics. In this paper, the consequences of selecting an erroneous kinetic model on lifetime predictions is discussed. It is demonstrated, using both simulated and experimental data, that any kinetic analysis of a chain scission driven reaction performed assuming a first-order model entails enormous deviations in predictions. This occurs despite the fact that the first-order kinetic model can fit experimental data from chain scission driven reactions with significant correlation coefficients, and even lead to a reasonably good reconstruction of the original experimental curves.
Febrero, 2022 | DOI: 10.1007/s10973-021-10649-x
Materiales de Diseño para la Energía y Medioambiente
Iron-catalyzed graphitization for the synthesis of nanostructured graphitic carbons
Hunter, RD; Ramirez-Rico, J; Schnepp, ZJournal of Materials Chemistry A, 10 (2022) 4489-4516
Show abstract ▽
Carbons are versatile and diverse materials that have numerous applications across energy and environmental sciences. Carbons with a graphitic structure are particularly appealing due to their high chemical stability, large surface areas and high thermal and electronic conductivity. Numerous methods exist to produce nanostructured graphitic carbons but some of these can be energy-intensive and/or have problems with scalability. One option that is being increasingly explored is the process of iron-catalyzed graphitization. This simply involves the pyrolysis of carbon-rich precursors in the presence of an iron catalyst and has been used to produce carbons with a wide range of structures and properties. This review will examine the current field of iron-catalyzed graphitization, with a focus on molecular organic or biomass precursors. Bio-derived precursors are particularly attractive as a potential option for sustainable production of graphitic carbons. We start with a brief introduction to some key carbon structures, the current applications in which they are employed and some of the key methods that have been developed to produce nanostructured graphitic carbons. We will then review the history of catalytic graphitization before evaluating the wide range of conditions and precursors that have been employed in catalytic graphitization. Finally, this review will investigate the current challenges facing iron-catalyzed graphitization, looking particularly at the limitations of the current understanding of the mechanistic aspects of graphitization, with a view to outlining where research in this field might progress.
Febrero, 2022 | DOI: 10.1039/d1ta09654k
Nanotecnología en Superficies y Plasma
Plasma assisted CO2 dissociation in pure and gas mixture streams with a ferroelectric packed-bed reactor in ambient conditions
Navascues, P; Cotrino, J; Gonzalez-Elipe, AR; Gomez-Ramirez, AChemical Engineering Journal, 430 (2022) 133066
Show abstract ▽
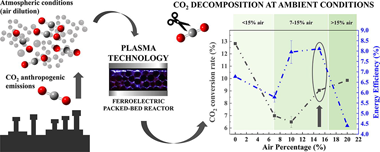
Carbon dioxide decomposition is a challenging target to combat climate change. Nonthermal plasmas are advantageous for this purpose because they operate at ambient conditions and can be easily scaled-up. In this study, we attempt the CO2 splitting into CO and O-2 in a parallel plate packed-bed plasma reactor moderated with Lead Zirconate Titanate (PZT) as fermelectric component, achieving conversion rates and energy efficiencies higher than those obtained with BaTiO3 in our experimental device. The analysis of the reaction mechanisms with optical emission spectroscopy under various operating conditions has shown a direct correlation between energy efficiency and intensity of CO* emission bands. These results and those obtained with a LiNbO3 plate placed onto the active electrode suggest that high temperature electrons contribute to the splitting of CO2 through an enhancement in the formation of CO2+ intermediate species. Results obtained for CO2 + O-2 mixtures confirm this view and suggest that back recombination processes involving CO and O-2 may reduce the overall splitting efficiency. The study of mixtures of CO2 and dry air has proved the capacity of fermelectric packed-bed reactors to efficiently decompose CO2 with no formation of harmful NxOy subproducts in conditions close to those in real facilities. The found enhancement in energy efficiency with respect to that found for the pure gas decomposition supports that new reaction pathways involving nitrogen molecules are contributing to the dissociation reaction. We conclude that PZT moderated packed-bed plasma reactors is an optimum alternative for the decompositon of CO2 in real gas flows and ambient conditions.
Febrero, 2022 | DOI: 10.1016/j.cej.2021.133066
- ‹ anterior
- 57 of 410
- siguiente ›














