Scientific Papers in SCI
2019
2019
Reactividad de Sólidos
The Calcium-Looping (CaCO3/CaO) process for thermochemical energy storage in Concentrating Solar Power plants
Ortiz, C; Valverde, JM; Chacartegui, R; Perez-Maqueda, LA; Gimenez, PRenewable & Sustanaible Energy Reviews, 113 (2019) 109252
Show abstract ▽
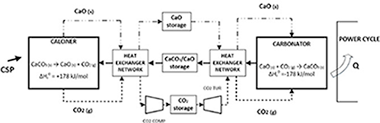
Energy storage based on thermochemical systems is gaining momentum as a potential alternative to molten salts in Concentrating Solar Power (CSP) plants. This work is a detailed review about the promising integration of a CaCO3/CaO based system, the so-called Calcium-Looping (CaL) process, in CSP plants with tower technology. The CaL process relies on low cost, widely available and non-toxic natural materials (such as limestone or dolomite), which are necessary conditions for the commercial expansion of any energy storage technology at large scale. A comprehensive analysis of the advantages and challenges to be faced for the process to reach a commercial scale is carried out. The review includes a deep overview of reaction mechanisms and process integration schemes proposed in the recent literature. Enhancing the multicycle CaO conversion is a major challenge of the CaL process. Many lab-scale analyses carried out show that residual effective CaO conversion is highly dependent on the process conditions and the CaO precursors used, reaching values in a wide range (0.07–0.82). The selection of the optimal operating conditions must be based on materials performance, process integration, technology and economics aspects. Global plant efficiencies over 45% (without considering solar-side losses) show the interest of the technology. Furthermore, the technological maturity and potential of the process is assessed. The direction towards which future works should be headed is discussed.
October, 2019 | DOI: 10.1016/j.rser.2019.109252
Nanotecnología en Superficies y Plasma
Influence of Titanium Oxide Pillar Array Nanometric Structures and Ultraviolet Irradiation on the Properties of the Surface of Dental Implants-A Pilot Study
Leon-Ramos, JR; Diosdado-Cano, JM; Lopez-Santos, C; Barranco, A; Torres-Lagares, D; Serrera-Figallo, MANanomaterials, 9 (2019) 1458
Show abstract ▽
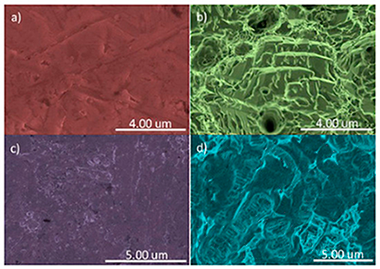
Aim: Titanium implants are commonly used as replacement therapy for lost teeth and much current research is focusing on the improvement of the chemical and physical properties of their surfaces in order to improve the osseointegration process. TiO2, when it is deposited in the form of pillar array nanometric structures, has photocatalytic properties and wet surface control, which, together with UV irradiation, provide it with superhydrophilic surfaces, which may be of interest for improving cell adhesion on the peri-implant surface. In this article, we address the influence of this type of surface treatment on type IV and type V titanium discs on their surface energy and cell growth on them. Materials and methods: Samples from titanium rods used for making dental implants were used. There were two types of samples: grade IV and grade V. In turn, within each grade, two types of samples were differentiated: untreated and treated with sand blasting and subjected to double acid etching. Synthesis of the film consisting of titanium oxide pillar array structures was carried out using plasma-enhanced chemical vapor deposition equipment. The plasma was generated in a quartz vessel by an external SLAN-1 microwave source with a frequency of 2.45 GHz. Five specimens from each group were used (40 discs in total). On the surfaces to be studied, the following determinations were carried out: (a) X-ray photoelectron spectroscopy, (b) scanning electron microscopy, (c) energy dispersive X-ray spectroscopy, (d) profilometry, (e) contact angle measurement or surface wettability, (f) progression of contact angle on applying ultraviolet irradiation, and (g) a biocompatibility test and cytotoxicity with cell cultures. Results: The application of ultraviolet light decreased the hydrophobicity of all the surfaces studied, although it did so to a greater extent on the surfaces with the studied modification applied, this being more evident in samples manufactured in grade V titanium. In samples made in grade IV titanium, this difference was less evident, and even in the sample manufactured with grade IV and SLA treatment, the application of the nanometric modification of the surface made the surface optically less active. Regarding cell growth, all the surfaces studied, grouped in relation to the presence or not of the nanometric treatment, showed similar growth. Conclusions. Treatment of titanium oxide surfaces with ultraviolet irradiation made them change temporarily into superhydrophilic ones, which confirms that their biocompatibility could be improved in this way, or at least be maintained.
October, 2019 | DOI: 10.3390/nano9101458
Química de Superficies y Catálisis
Effect of starch as binder in carbon aerogel and carbon xerogel preparation
Rodriguez, N; Agamez-Pertuz, YY; Romero, E; Diaz-Velasquez, JD; Odriozola, JA; Centeno, MAJournal of Non-Crystalline Solids, 522 (2019) UNSP 119554
Show abstract ▽
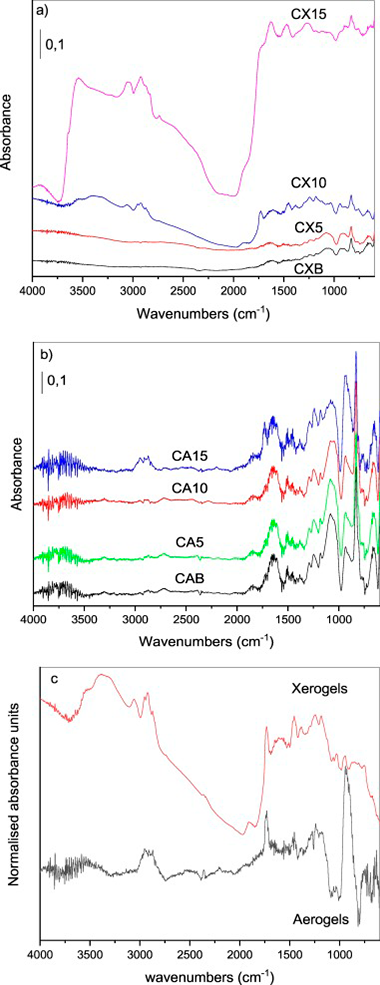
Carbon aerogels and carbon xerogels were synthesized through resorcinol - formaldehyde polycondensation using Na2CO3 as catalyst. The effect of soluble starch introduction in the organic gel preparation on the porous surface properties of these materials was studied. The role of the drying process of the organic gels on the changes in the surface and structural properties of these materials after the addition of soluble starch is discussed. The presence of starch in the prepared carbon xerogels results in the development of microporosity while maintaining the characteristic mesoporosity of carbon xerogels. The Brunauer - Emmett -Teller (BET) surface area increases from 309 m(2)/g in carbon xerogel without soluble starch until 685 m(2)/g when 10% of soluble starch is added. The R- value and average crystallite lattice parameters, inter-layer spacing, crystallite height, crystallite diameter and the average number of aromatic layers per carbon crystallite are discussed in function of drying step and presence of soluble starch. The surface properties were also studied by Raman and DRIFT spectroscopies.
October, 2019 | DOI: 10.1016/j.jnoncrysol.2019.119554
Nanotecnología en Superficies y Plasma
Highly selective few-ppm NO gas-sensing based on necklace-like nanofibers of ZnO/CdO n-n type I heterojunction
Naderi, H; Hajati, S; Ghaedi, M; Espinos, JPSensors and Actuators B-Chemical, 297 (2019) 126774
Show abstract ▽
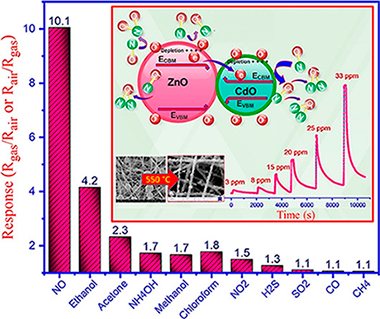
Electrospinning method followed by calcination is applied to synthesize ZnO/CdO nanofibers. Characterization is performed by X-ray diffraction (XRD), scanning electron microscopy (SEM), field emission scanning electron microscopy (FESEM), energy dispersive spectroscopy (EDS), diffuse reflectance spectroscopy (DRS), X-ray photoelectron spectroscopy (XPS), ultraviolet photoelectron spectroscopy (UPS) and reflection electron energy loss spectroscopy (REELS), which resulted in detailed analysis of the sensing material. For instance, it was found that the ZnO/CdO is n-n type I heterojunction which possesses straddling energy band gap, which could affect the mechanism of gas sensing. An electroless gold-plated interdigitated electrode with spacing 200 mu m is fabricated on alumina substrate to host the designed nanofibers being used as gas sensor. Gas-sensing activity of the heterojunction is investigated against NO, NO2, H2S, CH4, SO2 and CO in addition to VOCs such as ethanol, acetone, ammonia, methanol, and chloroform with high selectivity and response to NO gas by monitoring resistance changes. Detailed discussion on the mechanism of sensing is presented. The ZnO/CdO nanofibers are found to be highly sensitive to very low concentration range of NO gas (1.2-33 ppm) at optimal operating temperature of 215 degrees C. The influence of humidity (20-96%) on the sensor response was found to be ignorable. Additionally, good repeatability and long-term stability (45 days, every 5 days, SD = 0.7) was obtained for this sensor. Typically, short response times of 47 and 35 s are obtained versus 3 and 33 ppm of NO, respectively, making our sensor promisingly applicable for monitoring this toxic gas in polluting industries, metropolises and maybe in exhaled breath.
October, 2019 | DOI: 10.1016/j.snb.2019.126774
Fotocatálisis Heterogénea: Aplicaciones
Comparison of the effects generated by the dry-soft grinding and the photodeposition of Au and Pt processes on the visible light absorption and photoactivity of TiO2
Galeano, L; Valencia, S; Marin, JM; Restrepo, G; Navio, JA; Hidalgo, MCMaterials Research Express, 6 (2019) 1050d9
Show abstract ▽
The influence of dry-soft grinding and photodeposition of gold (Au) or platinum (Pt) in the improvement of the photoactivity of TiO2 synthesized by an integrated sol-gel and solvothermal method was studied. TiO2 was modified by a dry-soft grinding process in a planetary ball mill (TiO2(G)). Subsequently, Au or Pt particles were photodeposited in both unmodified TiO2 and TiO2(G) obtaining Au-TiO2, Pt-TiO2, Au-TiO2(G), and Pt-TiO2(G) materials. The photoactivity of the materials was evaluated in the phenol photodegradation under simulated solar radiation. Pt-TiO2 showed the greatest degree of photoactivity improvement in comparison with TiO2 and TiO2-P25. The dry-soft grinding process led to a high photocatalytic activity of TiO2(G) that was similar to Pt-TiO2 activity as consequence of a slight increase in the crystallinity in TiO2(G) due to an additional anatase formation in comparison with TiO2. However, further photocatalytic improvement in TiO2(G) were not achieved with the addition of Au or Pt. Therefore, the dry-soft grinding treatment and noble metal deposition led to similar improvements in the photocatalytic activity of TiO2 for phenol oxidation.
October, 2019 | DOI: 10.1088/2053-1591/ab4316
- ‹ previous
- 114 of 410
- next ›














