Scientific Papers in SCI
2022
2022
Materiales Ópticos Multifuncionales
Enhanced red-UC luminescence through Ce3+ co-doping in NaBiF4:Yb3+/Ho3+(Er3+)/Ce3+ phosphors prepared by ultrafast coprecipitation approach
Giordano, L; Du, H; Castaing, V; Luan, F; Guo, D; Viana, BOptical Materials X, 16 (2022) 100199
Show abstract ▽
Series of Yb3+/Ho3+(Er3+)/Ce3+ co-doped NaBiF4 phosphors were synthesized through an ultrafast co-precipitation reaction technique at room temperature. The effect of the Ce3+ ions on the crystal structure and upconversion (UC) luminescence properties of the studied samples were investigated in detail. FTIR and XPS demonstrated the pre-formation of NaBiF4 and the introduction of Yb3+, Ho3+, Er3+ and Ce3+ all as dopants in the host materials. Under 980 nm excitation, NaBiF4:Yb3+,Ho3+(Er3+),Ce3+performed the characteristic emission of the activator ion, and the introduction of Ce3+ did not change the emission wavelengths, only the relative intensities. Due to partial good energy overlap when 2F7/2 Ce3+ manifold is populated, raising Ce3+ ions concentration enhanced the red UC emission versus green UC emission but also lead to significant decrease in the average lifetimes of all monitored emissions for Ho3+ and Er3+. These lifetime decreases are explained by the energy loss in non-radiative pathways after the introduction of Ce3+. In addition, the green to yellow color emission change through addition of Ce3+ was explored in NaBiF4: Yb3+,Ho3+,Ce3+ to propose a novel application in two-level anti-counterfeiting.
October, 2022 | DOI: 10.1016/j.omx.2022.100199
Química de Superficies y Catálisis
Ni-Phosphide catalysts as versatile systems for gas-phase CO2 conversion: Impact of the support and evidences of structure-sensitivity
Zhang, Q; Pastor-Perez, L; Villora-Pico, JJ; Joyce, M; Sepulveda-Escribano, A; Duyar, MS; Reina, TRFuel, 323 (2022) 124301
Show abstract ▽
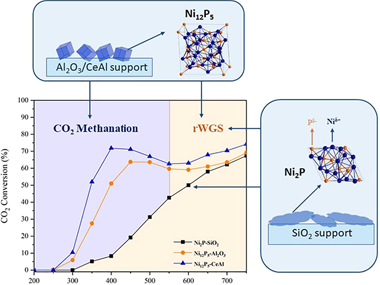
We report for the first time the support dependent activity and selectivity of Ni-rich nickel phosphide catalysts for CO2 hydrogenation. New catalysts for CO2 hydrogenation are needed to commercialise the reverse water-gas shift reaction (RWGS) which can feed captured carbon as feedstock for traditionally fossil fuel-based processes, as well as to develop flexible power-to-gas schemes that can synthesise chemicals on demand using surplus renewable energy and captured CO2. Here we show that Ni2P/SiO2 is a highly selective catalyst for RWGS, producing over 80% CO in the full temperature range of 350-750 degrees C. This indicates a high degree of suppression of the methanation reaction by phosphide formation, as Ni catalysts are known for their high methanation activity. This is shown to not simply be a site blocking effect, but to arise from the formation of a new more active site for RWGS. When supported on Al2O3 or CeAl, the dominant phase of as synthesized catalysts is Ni12P5. These Ni12P5 catalysts behave very differently compared to Ni2P/SiO2, and show activity for methanation at low temperatures with a switchover to RWGS at higher temperatures (reaching or approaching thermodynamic equilibrium behaviour). This switchable activity is interesting for applications where flexibility in distributed chemicals production from captured CO2 can be desirable. Both Ni12P5/Al2O3 and Ni12P5/CeAl show excellent stability over 100 h on stream, where they switch between methanation and RWGS reactions at 50-70% conversion. Catalysts are characterized before and after reactions via X-ray Diffraction (XRD), X-ray Photoelectron Spectroscopy (XPS), temperature-programmed reduction and oxidation (TPR, TPO), Transmission Electron Microscopy (TEM), and BET surface area measurement. After reaction, Ni2P/SiO(2 )shows the emergence of a crystalline Ni12P5 phase while Ni12P5/Al2O3 and Ni12P5/CeAl both show the crystalline Ni3P phase. While stable activity of the latter catalysts is demonstrated via extended testing, this Ni enrichment in all phosphide catalysts shows the dynamic nature of the catalysts during operation. Moreover, it demonstrates that both the support and the phosphide phase play a key role in determining selectivity towards CO or CH4.
September, 2022 | DOI: 10.1016/j.fuel.2022.124301
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Unraveling the Mo/HZSM-5 reduction pre-treatment effect on methane dehydroaromatization reaction
Lopez-Martin, A; Caballero, A; Colon, GApplied Catalysis B-Environmental, 312 (2022) 121382
Show abstract ▽
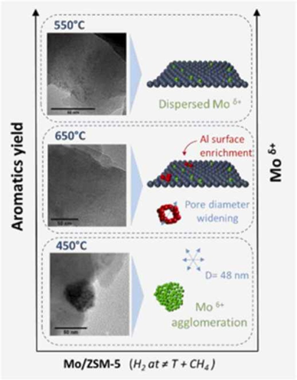
Reduction pre-treatment at different temperatures were performed over Mo/HZSM-5 system before methane dehydroaromatiztion reaction. We have shown the crucial effect of reduction temperature on the final catalytic performance. Outstanding improvement in the aromatics conversion has been attained. Thus, H-2 formation form methane cracking reaction seems to be hindered for pre-treated catalysts. As a consequence, the deposition of coke in these samples appeared also notably suppressed. The optimum performance has been achieved for reduction pre-treatment at 550 degrees C. For this temperature, we have observed that the fraction of reduced Mo species is higher.
September, 2022 | DOI: 10.1016/j.apcatb.2022.121382
Química de Superficies y Catálisis
Sustainable routes for acetic acid production: Traditional processes vs a low-carbon, biogas-based strategy
Martin-Espejo, JL; Gandara-Loe, J; Odriozola, JA; Reima, TR; Pastor-Pérez, LScience of the Total Environment, 840 (2022) 156663
Show abstract ▽
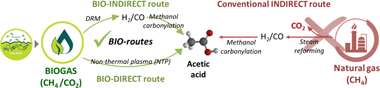
The conversion of biogas, mainly formed of CO2 and CH4, into high-value platform chemicals is increasing attention in a context of low-carbon societies. In this new paradigm, acetic acid (AA) is deemed as an interesting product for the chemical industry. Herein we present a fresh overview of the current manufacturing approaches, compared to potential low-carbon alternatives. The use of biogas as primary feedstock to produce acetic acid is an auspicious alternative, representing a step-ahead on carbon-neutral industrial processes. Within the spirit of a circular economy, we propose and analyse a new BIO-strategy with two noteworthy pathways to potentially lower the environmental impact. The generation of syngas via dry reforming (DRM) combined with CO2 utilisation offers a way to produce acetic acid in a two-step approach (BIO-Indirect route), replacing the conventional, petroleum-derived steam reforming process. The most recent advances on catalyst design and technology are discussed. On the other hand, the BIO-Direct route offers a ground-breaking, atom-efficient way to directly generate acetic acid from biogas. Nevertheless, due to thermodynamic restrictions, the use of plasma technology is needed to directly produce acetic acid. This very promising approach is still in an early stage. Particularly, progress in catalyst design is mandatory to enable low-carbon routes for acetic acid production.
September, 2022 | DOI: 10.1016/j.scitotenv.2022.156663
Química de Superficies y Catálisis
Hydrogen production from landfill biogas: Profitability analysis of a real case study
Vidal-Barrero, F; Baena-Moreno, FM; Preciado-Cardenas, C; Villanueva-Perales, A; Reina, TRFuel, 324 (2022) 124438
Show abstract ▽
Hydrogen is not only considered as a cornerstone within renewable energy portfolio but it is also a key enabler for CO2 valorisation being a central resource for industrial decarbonization. This work evaluates the profitability of hydrogen production via combined biogas reforming and water-gas shift reaction, based on a real case scenario for landfill biogas plant in Seville (Spain). A techno-economic model was developed based on a process model and the discounted cash-flow method. A biogas flow of 700 m(3)/h (input given by the landfill biogas plant) was used as plant size and the analysis was carried out for two different cases: (1) use of already available energy sources at the industrial plant, and (2) solar energy generation to power the process. The economic outputs obtained showed that under the current circumstances, this hydrogen production route is not profitable. The main reason is the relatively low current hydrogen prices which comes from fossil fuels. A revenues analysis indicates that hydrogen from biogas selling prices between 2.9 and 5.7 euro/kg would be needed to reach profitability, which are considerably higher than the current hydrogen cost (1.7 euro/kg). A subsidy scheme is suggested to improve the competitiveness of this hydrogen production process in the short-medium term. A cost analysis is also performed, revealing that electricity prices and investment costs have a high impact on the total share (23-40% and 8-22%, respectively). Other potential costs reduction such as catalyst, labour and manteinance & overhead are also evaluated, showing that cutting-down production costs is mandatory to unlock the potential of hydrogen generation from biogas. Our work showcases the techno-economic challenge that green energy policies face in the path toward sustainable societies.
September, 2022 | DOI: 10.1016/j.fuel.2022.124438
- ‹ previous
- 42 of 412
- next ›














