Artículos SCI
2024
2024
Química de Superficies y Catálisis
Switchable catalysis for methanol and synthetic natural gas synthesis from CO2: A techno-economic investigation
Merkouri, LP; Mathew, J; Jacob, J; Reina, TR; Duyar, MSJournal of CO2 Utilization, 79 (2024) 102652
Show abstract ▽
The oil and gas sector produces a considerable volume of greenhouse gas emissions, mainly generated from flaring and venting natural gas. Herein, a techno-economic analysis has been performed of a switchable catalytic process to convert the CH4 and CO2 in flared/vented natural gas into syngas or methanol. Specifically, it was shown that depending on greenhouse gas composition, dry methane reforming (DRM), reverse water-gas shift (RWGS), and CO2 methanation could be chosen to valorise emissions in an overall profitable and flexible operation scenario. The switchable process produced methanol and synthetic natural gas as its products, resulting in an annual income of €687m and annual operating expenses of €452m. The pre-tax profit was calculated at €234m, and at the end of the project, the net present value was calculated as €1.9b with a profitability index of 4.7€/€. The expected payback time of this process was ca. 4 years, and with a 35% internal rate of return (IRR). Most importantly, this process consumed 42.8m tonnes of CO2 annually. The sensitivity analysis revealed that variations in operation time, green hydrogen price, and products' prices significantly impacted the profitability of the process. Overall, this techno-economic analysis demonstrated that switchable catalysis in greenhouse gas utilisation processes is profitable, and thus it could play an important role in achieving net zero emissions.
Enero, 2024 | DOI: 10.1016/j.jcou.2023.102652
Fotocatálisis Heterogénea: Aplicaciones
Evaluation of Pt/TiO2-Nb2O5 systems in the photocatalytic reforming of glucose for the generation of H2 from industrial effluents
Lara Sandoval, AE; Serafin, J; Murcia Mesa, JJ; Rojas Sarmiento, HA; Hernandez Niño, JS; Llorca, J; Navío Santos, JA; Hidalgo Lõpez; MCFuel, 363 (2024) 130932
Show abstract ▽
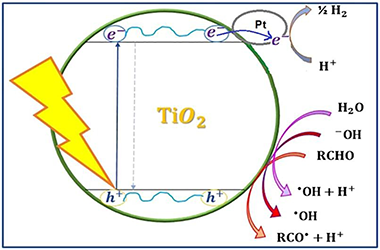
Different Pt-TiO2-Nb2O5 systems were synthesized and studied in the photocatalytic reforming of glucose for the generation of H2. The physicochemical properties of the synthesized photocatalysts were analyzed using different characterization techniques from which it was found that fluoridation and sulphation have different effects on the oxides under study such as a protective effect on the crystalline phase in anatase, and greater response in the visible region of the electromagnetic spectrum. The addition of fluorine or sulfates favors the reduction of platinum species on the surface of the semiconductor oxides and a better homogeneity of size and distribution of the particles of this metal. Studies were carried out in the gas phase that allowed the monitoring and quantification of the hydrogen produced from aqueous glucose solutions and it was determined that Pt-F-Nb2O5 and Pt-F-TiO2 are the most efficient materials for the production of hydrogen from this substrate. Similarly, liquid phase studies were carried out with a real sample from a confectionery industry where it was determined that with the material Pt-F-Nb2O5 the highest transformation of glucose is obtained, without the formation of any other sugar or intermediate compound, indicating the preferential production of hydrogen during the photocatalytic reaction. The foregoing demonstrates the potential of the evaluated process in obtaining this gas from the recovery of polluting residues derived from the samples under study.
Mayo, 2024 | DOI: 10.1016/j.fuel.2024.130932
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
CuO-TiO2 pilot-plant system performance for solar photocatalytic hydrogen production
Villachica-Llamosas, JG; Ruiz-Aguirre, A; Colón, G; Peral, J; Malato, SInternational Journal of Hydrogen Energy, 51 (2024) 1069-1077
Show abstract ▽
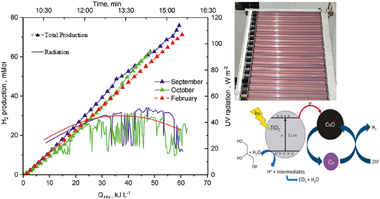
The main goal of the present study was to explore photocatalytic performance of the TiO2 -CuO mixture, for solar to hydrogen conversion at pilot plant scale under two different irradiation conditions (sunny and partly cloudy), focusing on high-temperature pretreat-ment of the catalyst mixture to try to improve TiO2 doping with copper. P25-TiO2 and commercial CuO were used with different amounts of Cu (2 wt% or 7 wt% Cu) calcined at 200-400 degrees C during several hours. Catalysts were tested at pilot plant scale using solar compound parabolic collectors, with glycerol as the sacrificial agent. The photocatalyst prepared after heating at 200 degrees C for 3 h and with 7 wt% Cu, resulted in higher hydrogen production than under the other heating conditions, and results were slightly better (5 -10%) than the reference values with the untreated catalysts. Photocatalytic efficiency was slightly lower at the higher calcination temperature (400 degrees C). CO2 production and formation of formate and glycolate clearly demonstrated glycerol photoreforming. The Cu from the calcined catalyst remaining on the solid was significantly less (2.5%) than on the non -calcined catalyst (4.2%), with an important fraction of lixiviated copper and copper deposition on the reactor walls. This is a critical drawback that must be considered for large-scale applications.
Enero, 2024 | DOI: 10.1016/j.ijhydene.2023.07.149
Nanotecnología en Superficies y Plasma
Green hydrogen production using doped Fe2O3 foams
Damizia, M; Lloreda-Jurado, PJ; De Filippis, P; de Caprariis, B; Chicardi, E; Sepúlveda, RInternational Journal of Hydrogen Energy, 51 (2024) 834-845
Show abstract ▽
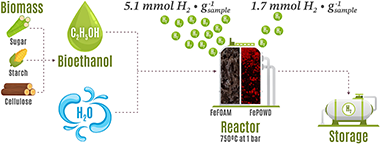
Hydrogen is the ideal energy vector to reduce our fossil-fuels dependency and diminish the climate change consequence. However, current production is still methane based. It is possible to produce hydrogen using bioethanol from the alcoholic fermentation of organic waste by chemical looping processes, but unfortunately current redox systems generate hydrogen with significant traces of CO. In the case of proton exchange membrane fuel cells (PEMFC), hydrogen must be highly purified to produce electricity. Here, high porosity inter-connected Fe2O3 foams doped with 2 wt% Al2O3 were manufactured by the freeze-casting method, obtaining around 5.1 mmol H2$g?1 sample of highly pure hydrogen (<10 ppm of CO) consuming only 3.42 mmol of ethanol on each redox cycles, with no deactivation. This result shows the possibility of using an abundant and inexpensive raw material as the iron oxide to scale-up the direct pure H2 production and facilitates its use in the automotive sector.
Enero, 2024 | DOI: 10.1016/j.ijhydene.2023.09.008
Química de Superficies y Catálisis
A profitability study for catalytic ammonia production from renewable landfill biogas: Charting a route for the next generation of green ammonia
González-Arias, J; Nawaz, MA; Vidal-Barrero, F; Reina, TRFuel, 360 (2024) 130584
Show abstract ▽
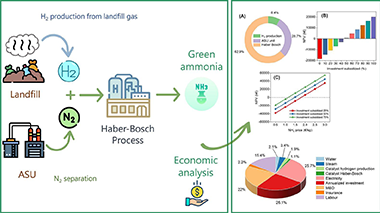
This study introduces a novel techno-economic approach to renewable ammonia production using landfill biogas. The proposed process involves bio-hydrogen generation from landfill biogas, nitrogen production via air separation, and the Haber-Bosch process. Building on our prior research, which demonstrated the economic competitiveness of renewable hydrogen production from landfill gas, we extend our investigation to analyze the feasibility of producing renewable ammonia from biogas-derived bio-hydrogen. However, the economic analysis for the baseline scenario reveals the current lack of profitability (net present value of −18.3 M€), with ammonia prices needing to quadruple to achieve profitability. Major costs, including investment, maintenance, overhead expenses, and electricity, collectively account for over 70%, suggesting the potential efficacy of investment subsidies as a political tool. Only cases with subsidies exceeding 50% of total investment costs, under current ammonia market prices, would render the green ammonia route profitable. Our findings underscore the significant techno-economic challenges in realizing renewable ammonia production, emphasizing the need for innovation in process engineering and catalytic technologies to enable competitive and scalable green ammonia production.
Marzo, 2024 | DOI: 10.1016/j.fuel.2023.130584
- ‹ anterior
- 3 of 410
- siguiente ›
icms











