Artículos SCI
2024
2024
Materiales Avanzados
Synthesis and characterization of porous and photocatalytic geopolymers based on natural clay: Enhanced properties and efficient Rhodamine B decomposition
Ettahiri, Y; Bouna, L: Brahim, A; Benlhachemi, A; Bakiz, B; Sánchez-Soto, PJ; Eliche-Quesada, D; Pérez-Villarejo, LApplied Materials Today, 36 (2024) 102048
Show abstract ▽
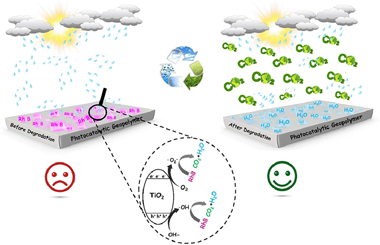
In this work, the incorporation of anatase TiO2 semiconductor in the geopolymer matrix as catalytic materials has been studied. The most noteworthy results obtained from the synthesis of a novel TiO2/geopolymer nanocomposite as an effective ecological catalyst with high thermal stability and significant porosity is presented. The porous and photocatalytic geopolymers based natural clay rich in pyrophyllite and kaolinite minerals were prepared by simple method, the geopolymerization reaction was able to successfully load TiO2 nanoparticles into the geopolymer surface. Furthermore, the results indicate that the prepared catalyst achieved the best performance to degrade Rhodamine B (RhB) molecules present in aqueous solution under UV light irradiation. The geopolymer matrix proved to be a reusable support for TiO2 nanoparticles during the photocatalytic process, efficiently facilitating the separation of photogenerated charges. Finally, the physicochemical and morphological properties of the samples was characterized by several techniques, namely X-ray Fluorescence (XRF), X-ray diffraction (XRD), Fourier Transform Infrared spectroscopy (FTIR), Thermogravimetric and Differential Thermal Analysis (TGA/DTA), N2 adsorption/desorption isotherm analysis (BET and BJH methods), UV–Vis Diffuse Reflectance Spectroscopy (DRS), Scanning Electron Microscopy (SEM) coupled to an Energy Dispersive X-ray Spectroscopy (EDS) analyzer and Transmission Electron Microscopy (TEM).
Febrero, 2024 | DOI: 10.1016/j.apmt.2023.102048
Química de Superficies y Catálisis
Reforming of biomass-derived producer gas using toluene as model tar: Deactivation and regeneration studies in Ni and K-Ni catalysts
Azancot, L; González-Castaño, M; Bobadilla, LF; Centeno, MA; Odriozola, JAEnvironmental Research, 247 (2024) 118210
Show abstract ▽
Within the syngas production from biomass gasification, tar removal constitutes a chief issue to overcome for advanced catalytic systems. This work investigates the performance of Ni and Ni-K catalysts for reforming of derived-biomass producer gas using toluene as model tar. At 750 degrees C and 60Lg(-1)h(-1), the stability test (70 h) revealed stable performances (CO2, CH4 and C7H8 conversions of 60, 95 and 100%, correspondingly) uniquely for the Ni-K catalyst. Although the efficient protection towards coking let by K was demonstrated, TPO studies over the post-reacted systems still evidenced the presence of carbon deposits for both samples. Conducting three successive reaction/regeneration cycles with different gasifying agents (air, steam and CO2) at 800 C for 1h, the capability towards regeneration of both catalytic systems was assessed and the spent catalysts were characterized by XRD, SEM and TEM. While none of the regeneration treatments recovered the performance of the unpromoted catalyst, the Ni-K catalysts demonstrated the capability of being fully regenerated by air and CO2 and exhibited analogous catalytic performances after a series of reaction/regeneration cycles. Hence, it is proved that the addition of K into Ni catalysts not only enhances the resistance against deactivation but enables rather facile regenerative procedures under certain atmospheres (air and CO2).
Abril, 2024 | DOI: 10.1016/j.envres.2024.118210
Química de Superficies y Catálisis
A review on high-pressure heterogeneous catalytic processes for gas-phase CO2 valorization
Villora-Picó, J.J; González-Arias, J; Pastor-Pérez, L; Odriozola, JA; Reina, TREnvironmental Research, 240 (2024) 117520
Show abstract ▽
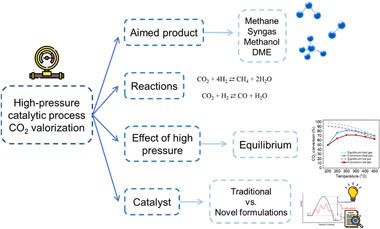
This review discusses the importance of mitigating CO2 emissions by valorizing CO2 through high-pressure catalytic processes. It focuses on various key processes, including CO2 methanation, reverse water-gas shift, methane dry reforming, methanol, and dimethyl ether synthesis, emphasizing pros and cons of high-pressure operation. CO2 methanation, methanol synthesis, and dimethyl ether synthesis reactions are thermodynami-cally favored under high-pressure conditions. However, in the case of methane dry reforming and reverse water -gas shift, applying high pressure, results in decreased selectivity toward desired products and an increase in coke production, which can be detrimental to both the catalyst and the reaction system. Nevertheless, high-pressure utilization proves industrially advantageous for cost reduction when these processes are integrated with Fischer-Tropsch or methanol synthesis units. This review also compiles recent advances in heterogeneous catalysts design for high-pressure applications. By examining the impact of pressure on CO2 valorization and the state of the art, this work contributes to improving scientific understanding and optimizing these processes for sustainable CO2 management, as well as addressing challenges in high-pressure CO2 valorization that are crucial for industrial scaling-up. This includes the development of cost-effective and robust reactor materials and the development of low-cost catalysts that yield improved selectivity and long-term stability under realistic working environments.
Enero, 2024 | DOI: 10.1016/j.envres.2023.117520
Química de Superficies y Catálisis
Optimizing biogas methanation over nickel supported on ceria-alumina catalyst: Towards CO2-rich biomass utilization for a negative emissions society
González-Arias, J; Torres-Sempere, G; Arroyo-Torralvo, F; Reina, TR; Odriozola, JAEnrironmental Research, 242 (2024) 117735
Show abstract ▽
Biogas methanation emerges as a prominent technology for converting biogas into biomethane in a single step. Furthermore, this technology can be implemented at biogas plant locations, supporting local economies and reducing dependence on large energy producers. However, there is a lack of comprehensive studies on biogas methanation, particularly regarding the technical optimization of operational parameters and the profitability analysis of the overall process. To address this gap, our study represents a seminal work on the technical optimization of biogas methanation obtaining an empirical model to predict the performance of biogas methanation. We investigate the influence of operational parameters, such as reaction temperature, H2/CO2 ratio, space velocity, and CO2 share in the biogas stream through an experimental design. Based on previous research we selected a nickel supported on ceria-alumina catalyst; being nickel a benchmark system for methanation process such selection permits a reliable data extrapolation to commercial units. We showcase the remarkable impact of studied key operation parameters, being the temperature, the most critical factor affecting the reaction performance (ca. 2 to 5 times higher than the second most influencing parameter). The impact of the H2/CO2 ratio is also noticeable. The response surfaces and contour maps suggest that a temperature between 350 and 450 degrees C and an H2/CO2 ratio between 2.5 and 3.2 optimize the reaction performance. Further experimental tests were performed for model validation and optimization leading to a reliable predictive model. Overall, this study provides validated equations for technology scaling-up and techno-economic analysis, thus representing a step ahead towards real-world applications for bio-methane production.
Febrero, 2024 | DOI: 10.1016/j.envres.2023.117735
Química de Superficies y Catálisis
Switchable catalysis for methanol and synthetic natural gas synthesis from CO2: A techno-economic investigation
Merkouri, LP; Mathew, J; Jacob, J; Reina, TR; Duyar, MSJournal of CO2 Utilization, 79 (2024) 102652
Show abstract ▽
The oil and gas sector produces a considerable volume of greenhouse gas emissions, mainly generated from flaring and venting natural gas. Herein, a techno-economic analysis has been performed of a switchable catalytic process to convert the CH4 and CO2 in flared/vented natural gas into syngas or methanol. Specifically, it was shown that depending on greenhouse gas composition, dry methane reforming (DRM), reverse water-gas shift (RWGS), and CO2 methanation could be chosen to valorise emissions in an overall profitable and flexible operation scenario. The switchable process produced methanol and synthetic natural gas as its products, resulting in an annual income of €687m and annual operating expenses of €452m. The pre-tax profit was calculated at €234m, and at the end of the project, the net present value was calculated as €1.9b with a profitability index of 4.7€/€. The expected payback time of this process was ca. 4 years, and with a 35% internal rate of return (IRR). Most importantly, this process consumed 42.8m tonnes of CO2 annually. The sensitivity analysis revealed that variations in operation time, green hydrogen price, and products' prices significantly impacted the profitability of the process. Overall, this techno-economic analysis demonstrated that switchable catalysis in greenhouse gas utilisation processes is profitable, and thus it could play an important role in achieving net zero emissions.
Enero, 2024 | DOI: 10.1016/j.jcou.2023.102652
- ‹ anterior
- 2 of 410
- siguiente ›
icms











