Scientific Papers in SCI
2021
2021
Química de Superficies y Catálisis
Stepping toward Efficient Microreactors for CO2 Methanation: 3D-Printed Gyroid Geometry
Baena-Moreno, FM; Gonzalez-Castano, M; de Miguel, JCN; Miah, KUM; Ossenbrink, R; Odriozola, J.A.ACS Sustainable Chemistry & Engineering, 9 (2021) 8198-8206
Show abstract ▽
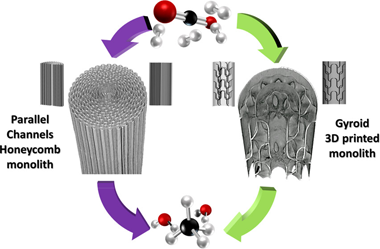
This work presents a comparative study toward the development of efficient microreactors based on three-dimensional (3D)-printed structures. Thus, the study evaluates the influence of the metal substrate geometry on the performance of structured catalysts for the CO2 methanation reaction. For this purpose, the 0.5%Ru-15%Ni/MgAl2O4 catalyst is washcoated over two different micromonolithic metal substrates: a conventional parallel channel honeycomb structure and a novel 3D-printed structure with a complex gyroid geometry. The effect of metal substrate geometry is analyzed for several CO2 sources including ideal flue gas atmospheres and the presence of residual CH4 and CO in the flue gas, as well as simulated biogas sources. The advantages of the gyroid 3D complex geometries over the honeycomb structures are shown for all evaluated conditions, providing in the best-case scenario a 14% improvement in CO2 conversion. Moreover, this contribution shows that systematically tailoring geometrical features of structured catalysts becomes an effective strategy to achieve improved catalyst performances independent of the flue gas composition. By enhancing the transport processes and the gas-catalyst interactions, the employed gyroid 3D metal substrates enable boosted CO2 conversions and greater CH4 selectivity within diffusion-controlled regimes.
June, 2021 | DOI: 10.1021/acssuschemeng.1c01980
Reactividad de Sólidos
Kinetic study of complex processes composed of non-independent stages: pyrolysis of natural rubber
Perejon, A; Sánchez-Jiménez, PE; García-Garrido, C; Pérez-Maqueda, LAPolymer Degradation and Stability, 188 (2021) 109590
Show abstract ▽
In this work, it is proposed a method for studying kinetics of complex processes composed of non-independent stages. In this method, the variable contribution of the different stages as a function of the heating schedule is taken into account. The method involves the simultaneous kinetic analysis of a set of experimental data registered under linear heating rate conditions, without any previous assumptions regarding the kinetic models followed by the stages or their corresponding activation energies.
The method has been tested with the kinetic analysis of the pyrolysis of natural rubber, since the kinetics of this process is complex and depends on temperature and heating schedule. It is demonstrated that the behavior of the experimental curves can be accurately predicted with the kinetic parameters calculated by the proposed methodology.
The kinetic analysis presented here could be applied to other complex processes as those found in pyrolysis, without the need of using oversimplified kinetic models that could yield significant errors when used in real applications.
June, 2021 | DOI: 10.1016/j.polymdegradstab.2021.109590
Química de Superficies y Catálisis
In-situ HDO of guaiacol over nitrogen-doped activated carbon supported nickel nanoparticles
Jin, Wei; Pastor-Perez, Laura; Villora-Pico, Juan J.; Mercedes Pastor-Blas, M.; Odriozola, Jose A.; Sepulveda-Escribano, Antonio; Ramirez Reina, TomasApplied Catalysis A-General, 620 (2021) 118033
Show abstract ▽
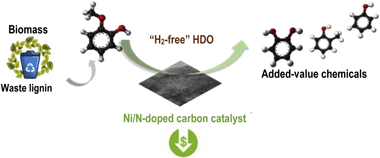
In-situ hydrodeoxygenation of guaiacol over Ni-based nitrogen-doped activated carbon supported catalysts is presented in this paper as an economically viable route for bio-resources upgrading. The overriding concept of this paper is to use water as hydrogen donor for the HDO reaction, suppressing the input of external highpressure hydrogen. The effect of nitrogen sources, including polypyrrole (PPy), polyaniline (PANI) and melamine (Mel) on the structural, electronic and ultimately of catalytic features of the designed materials have been addressed. Nitrogen-doped samples are more active than the undoped counterparts in the "H2-free" HDO process. For instance, the conversion of guaiacol increased by 8 % for Ni/PANI-AC compared to that of Ni/AC catalysts. The superior performance of Ni/NC can be attributed to the acid-base properties and modified electronic properties, which favours the C-O cleavage and water activation as well as enhances dispersion of Ni particles on the catalysts' surface.
June, 2021 | DOI: 10.1016/j.apcata.2021.118033
Materiales Ópticos Multifuncionales
The Role of the Atmosphere on the Photophysics of Ligand-Free Lead-Halide Perovskite Nanocrystals
Moran-Pedroso, M; Rubino, A; Calvo, ME; Espinos, JP; Galisteo-Lopez, JF; Miguez, HAdvanced Optical Materials, (2021) 2100605
Show abstract ▽
Lead halide perovskite (LHP) nanocrystals (NCs) have gained attention over the past decade due to their outstanding optoelectronic properties, making them a suitable material for efficient photovoltaic and light emitting devices. Due to its soft nature, these nanostructures undergo strong structural changes upon irradiation, where these light-induced processes are strongly influenced by the environment. Since most processing routes for LHP NCs are based on colloidal approaches, the role of factors such as stabilizing ligands or solvents is usually hard to disentangle from the interaction of external radiation with the perovskite material. Employing a recently proposed synthetic approach, where ligand-free NCs can be grown within metal-oxide-based insulating nanoporous matrices, it has been feasible to perform a clean study of the effect of the surrounding atmosphere on the photophysical properties of perovskite NCs, avoiding the interference of protective capping layers or solvents. Simultaneous light-induced photo-activation and darkening processes are monitored and disentangled, and their relation with bulk and surface processes, respectively, demonstrated.
June, 2021 | DOI: 10.1002/adom.202100605
Química de Superficies y Catálisis
Ni/YMnO3 perovskite catalyst for CO2 methanation
Gonzalez-Castano, M; de Miguel, JCN; Penkova, A; Centeno, MA; Odriozola, JA; Arellano-Garcia, HApplied Materials Today, 23 (2021) 101055
Show abstract ▽
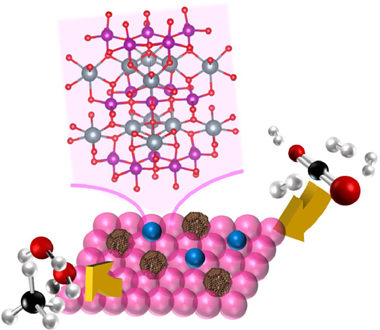
This work proposes an innovative Ni catalyst supported over YMnO3 perovskite as a promising catalytic system for CO2 methanation reaction. Under reductive conditions, the attendance of Mn redox couples within the layered perovskite structure promotes the constitution of sub-stoichiometric YMnO3-x units which, by means of the flexible YMnO3-x reorganization capacity, results in boosted anionic mobility's. The competitive turnover frequencies (20.1 and 17.0 s(-1) at 400 degrees C under dry- and steamed- CO2 methanation conditions) displayed by Ni/YMnO3 system were related to the synergism between strongly interacting Ni particles with partially reduced YMnO3-x perovskites. The optimal Ni dispersions, for which no relevant signs of sintering issues were discerned, combined to effective role of oxygen vacancies towards the dissociative activation of CO2 molecules enabled highly active and stable catalytic behaviours with no evidence of cooking phenomena. On evaluating the water presence within CO2 methanation feedstock's, the deprived catalytic behaviour was fundamentally associated to depleted oxygen vacancies concentrations and promoted WGS side reactions.
June, 2021 | DOI: 10.1016/j.apmt.2021.101055
- ‹ previous
- 74 of 410
- next ›














