Scientific Papers in SCI
2023
2023
Química de Superficies y Catálisis
Guaiacol hydrotreatment in an integrated APR-HDO process: Exploring the promoting effect of platinum on Ni-Pt catalysts and assessing methanol and glycerol as hydrogen sources
Jin, W; Gandara-Loe, J; Pastor-Perez, L; Villora-Pico, JJ; Sepulveda-Escribano, A; Rinaldi, R; Reina, TRRenewable Energy, 215 (2023) 118907
Show abstract ▽
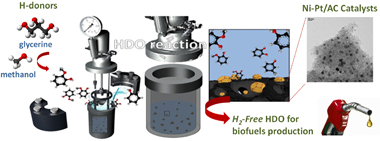
This study presents an integrated approach combining aqueous phase reforming (APR) and hydrodeoxygenation (HDO) for the hydrotreatment of guaiacol, a model compound representing lignin-derived phenols in pyrolysis bio-oils. The APR process enables in-situ H2 generation, eliminating the need for an external hydrogen source. We examine the interplay between metal species, the Pt-promoting effect on Ni-Pt catalyst supported on activated carbon (AC), and the choice of hydrogen source (methanol or glycerol). Amongst the monometallic catalysts, a 1% Pt/AC catalyst notably achieved over 96% guaiacol conversion at 300 degrees C with either hydrogen source. Interestingly, when 0.5-1% of the Ni loading is replaced with Pt, the resulting bimetallic Ni-Pt/AC catalysts demonstrate a significant improvement in guaiacol conversion, reaching 70% when methanol is employed as the hydrogen source. Surprisingly, no comparable enhancement in guaiacol conversion is observed when employing glycerol as the hydrogen source. This observation underlines one of the pivotal effects of the hydrogen source on catalyst performance. X-ray photoemission spectroscopy (XPS) pinpointed strong Ni-Pt interactions in the catalyst. It also revealed distinctive electronic features of Ni-Pt/AC, which are favourable for steering selectivity towards cyclohexanol rather than phenol when Pt loading is increased from 0.5 to 1%. Moreover, Pt enhanced catalyst stability by inhibiting the oxidation of Ni sites and mitigating Ni-Pt phase sintering. Overall, our findings offer important insights into integrating APR and HDO processes, the promotion effect of Pt, and the importance of hydrogen source selection in terms of guaiacol conversion and catalyst stability.
October, 2023 | DOI: 10.1016/j.renene.2023.118907
Reactividad de Sólidos
Influence of Long-Term CaO Storage Conditions on the Calcium Looping Thermochemical Reactivity
Amghar, N; Perejón, A; Ortiz, C; Maqueda, LAP; Sánchez-Jiménez, PEEnergy & Fuels, 37 (2023) 16904-16914
Show abstract ▽
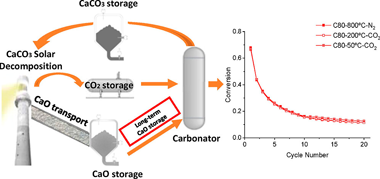
Long-term storage capability is often claimed as one of the distinct advantages of the calcium looping process as a potential thermochemical energy storage system for integration into solar power plants. However, the influence of storage conditions on the looping performance has seldom been evaluated experimentally. The storage conditions must be carefully considered as any potential carbonation at the CaO storage tank would reduce the energy released during the subsequent carbonation, thereby penalizing the round-trip efficiency. From lab-scale to conceptual process engineering, this work considers the effects of storing solids at low temperatures (50–200 °C) in a CO2 atmosphere or at high temperatures (800 °C) in N2. Experimental results show that carbonation at temperatures below 200 °C is limited; thus, the solids could be stored during long times even in CO2. It is also demonstrated at the lab scale that the multicycle performance is not substantially altered by storing the solids at low temperatures (under CO2) or high temperatures (N2 atmosphere). From an overall process perspective, keeping solids at high temperatures leads to easier heat integration, a better plant efficiency (+2–4%), and a significantly higher energy density (+40–62%) than considering low-temperature storage. The smooth difference in the overall plant efficiency with the temperature suggests a proper long-term energy storage performance if adequate energy integration is carried out.
October, 2023 | DOI: 10.1021/acs.energyfuels.3c02652
Materiales para Bioingeniería y Regeneración Tisular
Sol–Gel Technologies to Obtain Advanced Bioceramics for Dental Therapeutics
X. Song; J.J. Segura-Egea; A.Díaz-CuencaMolecules, 28 (2023) 6967
Show abstract ▽
The aim of this work is to review the application of bioceramic materials in the context of current regenerative dentistry therapies, focusing on the latest advances in the synthesis of advanced materials using the sol–gel methodology. Chemical synthesis, processing and therapeutic possibilities are discussed in a structured way, according to the three main types of ceramic materials used in regenerative dentistry: bioactive glasses and glass ceramics, calcium phosphates and calcium silicates. The morphology and chemical composition of these bioceramics play a crucial role in their biological properties and effectiveness in dental therapeutics. The goal is to understand their chemical, surface, mechanical and biological properties better and develop strategies to control their pore structure, shape, size and compositions. Over the past decades, bioceramic materials have provided excellent results in a wide variety of clinical applications related to hard tissue repair and regeneration. Characteristics, such as their similarity to the chemical composition of the mineral phase of bones and teeth, as well as the possibilities offered by the advances in nanotechnology, are driving the development of new biomimetic materials that are required in regenerative dentistry. The sol–gel technique is a method for producing synthetic bioceramics with high purity and homogeneity at the molecular scale and to control the surfaces, interfaces and porosity at the nanometric scale. The intrinsic nanoporosity of materials produced by the sol–gel technique correlates with the high specific surface area, reactivity and bioactivity of advanced bioceramics.
October, 2023 | DOI: 10.3390/molecules28196967
Materiales de Diseño para la Energía y Medioambiente
Sustainable Integration of Zinc Oxide Nanoparticles: Enhancing Properties of Poly(ε-Caprolactone) Electrospun Nanofibers and Cast Films
Abdullah, JAA; Benítez, JJ; Guerrero, A; Romero, ACoatings, 13 (2023) 1665
Show abstract ▽
This study investigated the impact of adding zinc oxide nanoparticles (ZnO-NPs) to electrospun membranes and cast films made of poly(epsilon-caprolactone) (PCL). The physicochemical, mechanical, and morphological properties of the samples were analyzed. Physicochemical parameters included water contact angle (WCA), water vapor transmission rate (WVTR), permeance, water vapor permeability (WVP), light transmission (T-600), and transparency (T). Mechanical properties, such as maximum stress (6(max)), elongation (epsilon(max)), and Young's modulus (MPa), were also evaluated. Morphological properties were analyzed in terms of thickness, dispersion, and surface roughness (measured by the arithmetic (Ra) and quadratic (Rq) averages). The crystallinity and melting point, as well as the functional DPPH center dot scavenging percentage (SP%), were also studied. The results showed that adding 1 wt% ZnO-NPs improved the water barrier properties of PCL membranes and films, increasing WCA by 1%-6% and decreasing WVTR by 11%-19%, permeance by 34%-20%, and WVP by 4%-11%, respectively. The T-600 values of PCL/ZnO-NPs membranes and films were 2-3 times lower than those of neat PCL samples, indicating improved optical properties. The mechanical properties of the composite membranes and films also improved, with 6(max) increasing by 56%-32% and Young's modulus increasing by 91%-95%, while epsilon(max) decreased by 79%-57%. The incorporation of ZnO-NPs also increased the thickness and surface roughness of the samples. The SP% of PCL/ZnO-NPs increased by almost 69%, demonstrating the beneficial effects of ZnO-NPs on the system. These findings suggest that incorporating ZnO-NPs into PCL membranes and films can enhance their properties, making them well suited for various applications, such as those within the realm of materials science and nanotechnology.
October, 2023 | DOI: 10.3390/coatings13101665
Fotocatálisis Heterogénea: Aplicaciones - Nanotecnología en Superficies y Plasma
Mechanistic aspects of the reduction of rutile titanium dioxide and its Re-oxidation. Development and destruction of crystallographic shear structures
Bickley, RI; Garside, GR; González-Carreño, T; González-Elipe, AR; Navío, JAJournal of Solid State Chemistry, 326 (2023) 124174
Show abstract ▽
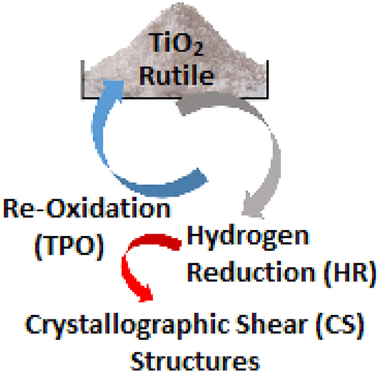
A model is presented giving the mean dimensions of acicular octadecahedral microcrystallites of a rutile titanium dioxide powder. Reduction at 823 K, in conjunction with ESR, electrical conductivity and controlled re-oxidation has enabled the model to be applied to reduced microcrystallites. At 300 K they contain <0.1% of paramagnetic [Ti3+↑ VO: ↑Ti3+] reduced edge sites and >99.9% of reduced spin-paired [Ti3+↑↓ Ti3+ VO:] sites. These sites are situated on the external crystal faces and on polygonal bulk crystallographic shear (CS) structures inclined to the microcrystal four-fold symmetry axis. CS structures are quantum-sized [Ti4O7VO:] environments which broaden the paramagnetic signals at 78 K. Temperature programmed reduction in H2(g) reveals atomic hydrogen as a precursor to CS structure formation via a lattice template formed on microcrystallite faces. Shear structures are oxidised on their polygonal perimeters at differing rates on the respective microcrystallite faces by anionic vacancy transfer from sub-surface regions.
October, 2023 | DOI: 10.1016/j.jssc.2023.124174
- ‹ previous
- 10 of 410
- next ›














