Scientific Papers in SCI
2021
2021
Propiedades mecánicas, modelización y caracterización de cerámicos avanzados
The Possible Detriment of Oxygen in Creep of Alumina and Zirconia Ceramic Composites Reinforced with Graphene
Cano-Crespo, R; Rivero-Antunez, P; Gomez-Garcia, D; Moreno, R; Dominguez-Rodriguez, AMaterials, 14 (2021) 984
Show abstract ▽
This paper aims to give an answer to the following question: is the oxidation of graphene a critical issue for high-temperature plasticity in graphene-reinforced ceramics? To give a convincing reply, we will focus on two very different graphene-based ceramic composites: reduced graphene oxide (rGO)-reinforced alumina (alpha-Al2O3) and reduced graphene oxide (rGO)-reinforced yttria tetragonal zirconia (t-ZrO2). The processing of the powders has been made using a colloidal route, and after that, a spark plasma sintering process was performed in order to densify the samples. Creep tests were performed at temperatures between 1200-1250 degrees C in an argon atmosphere. The microstructure obtained by SEM of the sintered and tested specimens was characterized quantitatively to elucidate the deformation mechanism. Raman spectroscopy was carried out to check the integrity of the graphene. The average grain size was in the order of 1 mu m and the shape factor was 0.7 for all the studied materials. The integrity of the graphene was checked before and after the creep experiments. The careful analysis of the creep tests shows that graphene oxide or its reduced version are not efficient phases for creep resistance improvement in general, contrary to what is reported elsewhere. However, the results permit the suggestion of a creep improvement in nanocomposites at a very high temperature regime due to an enhanced reactivity of oxygen between carbon and alumina interfaces. In the case of zirconia, the results give us the conclusion that the oxidation of graphene is a highly detrimental issue regarding the improvement of high-temperature plasticity.
February, 2021 | DOI: 10.3390/ma14040984
Reactividad de Sólidos
A Novel, Simple and Highly Efficient Route to Obtain PrBaMn2O5+δ Double Perovskite: Mechanochemical Synthesis
Garcia-Garcia, FJ; Sayagues, MJ; Gotor, FJNanomaterials, 11 (2021) 380
Show abstract ▽
In this work, a mechanochemical route was proposed for the synthesis of the PrBaMn2O5+δ (PMBO) double layered perovskite phase. The mechanochemical reaction between Pr6O11, BaO2, and MnO powders with cationic stoichiometric ratios of 1/1/2 for Pr/Ba/Mn was performed using high-energy milling conditions in air. After 150 min of milling, a new phase with perovskite structure and cubic symmetry consistent with the A-site disordered Pr0.5Ba0.5MnO3 phase was formed. When this new phase was subsequently annealed at a high temperature in an inert Ar atmosphere, the layered PrBaMn2O5+δ phase was obtained without needing to use a reducing atmosphere. At 1100 °C, the fully reduced layered PrBaMn2O5 phase was achieved. A weight gain was observed in the 200–300 °C temperature range when this fully reduced phase was annealed in air, which was consistent with the transformation into the fully oxidized PrBaMn2O6 phase. The microstructural characterization by SEM, TEM, and HRTEM ascertained the formation of the intended PrBaMn2O5+δ phase. Electrical characterization shows very high electrical conductivity of layered PBMO in a reducing atmosphere and suitable in an oxidizing atmosphere, becoming, therefore, excellent candidates as solid oxide fuel cell (SOFC electrodes).
February, 2021 | DOI: 10.3390/nano11020380
Fotocatálisis Heterogénea: Aplicaciones
Enhanced UV and visible light photocatalytic properties of synthesized AgBr/SnO2 composites
Puga, F.; Navío, J.A.; Hidalgo, M.C.Separation and Purification Tecnology, 257 (2021) 117948
Show abstract ▽
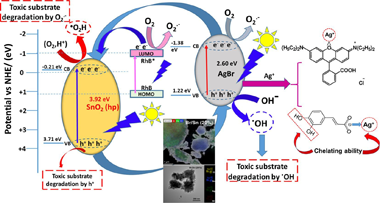
Composites (AgBr/SnO2) comprised of AgBr and SnO2 with different molar % of bare SnO2, have been synthesized by simple precipitation methods; the bare SnO2 used, was synthesized by hydrothermal procedure. Samples have been characterized by X-ray diffraction (XRD), N2-adsorption, UV–vis diffuse reflectance spectroscopy (DRS), scanning electron microscopy (SEM), Transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). Photocatalytic activity of the as-prepared photocatalysts was evaluated through photocatalytic degradation of rhodamine B (RhB) and caffeic acid (CAFA) under UV and Visible illumination. In photocatalytic degradation studies, for both substrates, conversion rates of around 95% were found in 45 min of both UV-illumination and 85% under visible lighting. These conversion rates were superior than the conversion rates of pure parental components, AgBr and SnO2 under the same experimental conditions. At least, for RhB no loss of photocatalytic activity has been observed after five recycles although the mineralization degree progressively diminished along the recycles. The enhanced photocatalytic degradation of AgBr/SnO2 compounds was attributed, in part, to a synergistic increase in adsorption viability, as well as to the effective separation of photoinduced load carriers that resulted from the formation of a heterojunction according to the type II junction. Radical scavengers’ experiments indicated that active oxidant species as O2.−, ·OH and h+ all are involved in this photocatalytic system, although it seems that O2.− played the major role in the photocatalytic degrading of RhB by AgBr/SnO2 composites. In summary, coupling AgBr with SnO2 remarkably improves the photocatalytic activity under both UV and visible-illumination with respect to the parental components. These features open the route to future applications of this material in the field of environmental remediation.
February, 2021 | DOI: 10.1016/j.seppur.2020.117948
Reactividad de Sólidos
Influence of Successive Chemical and Thermochemical Treatments on Surface Features of Ti6Al4V Samples Manufactured by SLM
Gonzalez, JE; de Armas, G; Negrin, J; Beltran, AM; Trueba, P; Gotor, FJ; Peon, E; Torres, YMetals, 11 (2021) 313
Show abstract ▽
Ti6Al4V samples, obtained by selective laser melting (SLM), were subjected to successive treatments: acid etching, chemical oxidation in hydrogen peroxide solution and thermochemical processing. The effect of temperature and time of acid etching on the surface roughness, morphology, topography and chemical and phase composition after the thermochemical treatment was studied. The surfaces were characterized by scanning electron microscopy, energy dispersive X-ray spectroscopy, X-ray diffraction and contact profilometry. The temperature used in the acid etching had a greater influence on the surface features of the samples than the time. Acid etching provided the original SLM surface with a new topography prior to oxidation and thermochemical treatments. A nanostructure was observed on the surfaces after the full process, both on their protrusions and pores previously formed during the acid etching. After the thermochemical treatment, the samples etched at 40 °C showed macrostructures with additional submicro and nanoscale topographies. When a temperature of 80 °C was used, the presence of micropores and a thicker anatase layer, detectable by X-ray diffraction, were also observed. These surfaces are expected to generate greater levels of bioactivity and high biomechanics fixation of implants as well as better resistance to fatigue.
February, 2021 | DOI: 10.3390/met11020313
Fotocatálisis Heterogénea: Aplicaciones
Sol-gel synthesis of ZnWO4-(ZnO) composite materials. Characterization and photocatalytic properties
Jaramillo-Páez, C., Navío, J.A., Puga, F., Hidalgo, M.C.Journal of Photochemistry & Photobiology, A: Chemistry, 404 (2021) 112962
Show abstract ▽
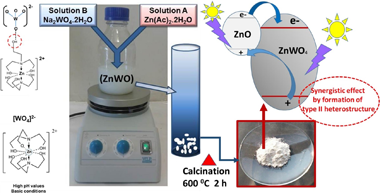
ZnWO4 based powder photocatalyst have been successfully prepared by calcining a co-precipitated precursor (ZnWO) obtained from aqueous Zn2+ and WO4 2− solutions at pH = 7, without surfactants addition. The as-formed sample was characterized by XRD, N2-absorption, SEM, TEM, DRS and XPS. Both techniques, XRD and XPS results showed that prepared sample corresponds to a crystalline, Zn-enriched composition, ZnWO4 indicating the formation of a ZnWO4-(ZnO) composite, whit ca. 10 wt.-% of ZnO confirmed by XRF analysis. Photocatalytic activities towards degradation of Rhodamine B (RhB), Methyl Orange (MO) and Phenol, under UV-illumination, was investigated not only by monitoring the percentages of conversion of substrates, but also by estimating the corresponding percentages of mineralization that accompany the photocatalytic process. Comparative substrateconversion rates estimated per surface area unit of catalyst, showed that the activity for ZnWO4-(ZnO) composite is similar to that for TiO2(P25), at least for MO and RhB, and even higher that for TiO2(P25) in respect to phenol conversion. By adding TEA to the synthesis procedure, a composite named as ZnWO4-ZnO-(pH = 10)-600 is generated, which has a higher proportion of ZnO (ca. 39 %) and superior specific surface area than the so-called ZnWO4-(ZnO) sample. Furthermore, the photocatalytic degradation of MO using the former material indicates that it is superior to ZnWO4-(ZnO) and even that TiO2(P25) itself under the same operational conditions.
January, 2021 | DOI: 10.1016/j.jphotochem.2020.112962
- ‹ previous
- 86 of 410
- next ›