Scientific Papers in SCI
2024
2024
Nanotecnología en Superficies y Plasma
Green hydrogen production using doped Fe2O3 foams
Damizia, M; Lloreda-Jurado, PJ; De Filippis, P; de Caprariis, B; Chicardi, E; Sepúlveda, RInternational Journal of Hydrogen Energy, 51 (2024) 834-845
Show abstract ▽
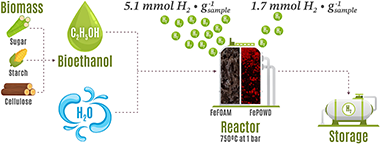
Hydrogen is the ideal energy vector to reduce our fossil-fuels dependency and diminish the climate change consequence. However, current production is still methane based. It is possible to produce hydrogen using bioethanol from the alcoholic fermentation of organic waste by chemical looping processes, but unfortunately current redox systems generate hydrogen with significant traces of CO. In the case of proton exchange membrane fuel cells (PEMFC), hydrogen must be highly purified to produce electricity. Here, high porosity inter-connected Fe2O3 foams doped with 2 wt% Al2O3 were manufactured by the freeze-casting method, obtaining around 5.1 mmol H2$g?1 sample of highly pure hydrogen (<10 ppm of CO) consuming only 3.42 mmol of ethanol on each redox cycles, with no deactivation. This result shows the possibility of using an abundant and inexpensive raw material as the iron oxide to scale-up the direct pure H2 production and facilitates its use in the automotive sector.
January, 2024 | DOI: 10.1016/j.ijhydene.2023.09.008
Química de Superficies y Catálisis
Natural hydrogen in the energy transition: Fundamentals, promise, and enigmas
Blay-Roger, R; Bach, W; Bobadilla, LF; Reina, TR; Odriozola, JA; Amils, R; Blay, VRenewable & Sustainable Energy Reviews, 189 (2024) 113888
Show abstract ▽
Beyond its role as an energy vector, a growing number of natural hydrogen sources and reservoirs are being discovered all over the globe, which could represent a clean energy source. Although the hydrogen amounts in reservoirs are uncertain, they could be vast, and they could help decarbonize energy-intensive economic sectors and facilitate the energy transition. Natural hydrogen is mainly produced through a geochemical process known as serpentinization, which involves the reaction of water with low-silica, ferrous minerals. In favorable locations, the hydrogen produced can become trapped by impermeable rocks on its way to the atmosphere, forming a reservoir. The safe exploitation of numerous natural hydrogen reservoirs seems feasible with current technology, and several demonstration plants are being commissioned. Natural hydrogen may show variable composition and require custom separation, purification, storage, and distribution facilities, depending on the location and intended use. By investing in research, in the mid-term, more hydrogen sources could become exploitable and geochemical processes could be artificially stimulated in new locations. In the long term, it may be possible to leverage or engineer the interplay between microorganisms and geological substrates to obtain hydrogen and other chemicals in a sustainable manner.
January, 2024 | DOI: 10.1016/j.rser.2023.113888
Fotocatálisis Heterogénea: Aplicaciones
Ba3(PO4)2 Photocatalyst for Efficient Photocatalytic Application
Naciri, Y; Ahdour, A; Benhsina, E; Hamza, MA; Bouziani, A; Hsini, A; Bakiz, B; Navio, JA; Ghazzal, MNGlobal Challenges
Show abstract ▽
Barium phosphate (Ba-3(PO4)(2)) is a class of material that has attracted significant attention thanks to its chemical stability and versatility. However, the use of Ba-3(PO4)(2) as a photocatalyst is scarcely reported, and its use as a photocatalyst has yet to be reported. Herein, Ba-3(PO4)(2) nanoflakes synthesis is optimized using sol-gel and hydrothermal methods. The as-prepared Ba-3(PO4)(2) powders are investigated using physicochemical characterizations, including XRD, SEM, EDX, FTIR, DRS, J-t, LSV, Mott-Schottky, and EIS. In addition, DFT calculations are performed to investigate the band structure. The oxidation capability of the photocatalysts is investigated depending on the synthesis method using rhodamine B (RhB) as a pollutant model. Both Ba-3(PO4)(2) samples prepared by the sol-gel and hydrothermal methods display high RhB photodegradation of 79% and 68%, respectively. The Ba-3(PO4)(2) obtained using the sol-gel process exhibits much higher stability under light excitation after four regeneration cycles. The photocatalytic oxidation mechanism is proposed based on the active species trapping experiments where O-2(center dot-) is the most reactive species. The finding shows the promising potential of Ba-3(PO4)(2) photocatalysts and opens the door for further investigation and application in various photocatalytic applications.
January, 2024 | DOI: 10.1002/gch2.202300257
2023
2023
Química de Superficies y Catálisis
Effect of zeolite topological structure in bifunctional catalyst on direct conversion of syngas to light olefins
Meng, FH; Gong, ZY; Yang, LL; Wang, Q; Xing, MQ; Nawaz, MA; Li, ZMicroporous and Mesoporous Materials, 362 (2023) 112792
Show abstract ▽
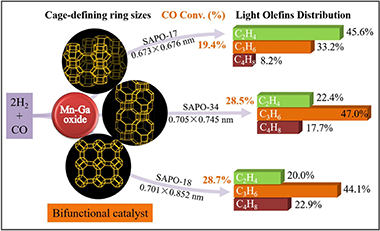
Bifunctional catalyst composed of metal oxide and zeolite (OX-ZEO) is a promising strategy for the direct conversion of syngas to light olefins (STO), where the structure of zeolite plays a vital role in determining the selectivity of product. Herein, three kinds of silicoaluminophosphate zeolites with different topological structures, i.e., the ERI(SP17), AEI(SP18) and CHA(SP34), were hydrothermally synthesized, after the combination with Mn-Ga oxide, the prepared OX-ZEO was applied for STO reaction. The variation in the crystallization time for SP17 synthesis has a great impact on the generation of impurity phase of SAPO-5, where a crystallization time of 48-96 h is found to be beneficial in synthesizing SP17 zeolite with pure phase. SP17 zeolite with a crystallization time of 96 h, possesses the micropores and columnar morphology, where the small cage-defining 8-ring size of SP17 shows the olefins selectivity of 87.0% at a low CO conversion of 19.4%, significantly deviating towards the major fraction of ethylene (45.6%) than that of butene (8.2%). In a contrast, SP18 and SP34 zeolites with the same and large cage-defining 8-ring size, are richer in propylene and butene fractions than that of ethylene in overall similar olefins selectivity of 87.0% and 87.1% at CO conversion of 28.7% and 28.5%, respectively. Interestingly, it is further interpreted that the SP17 sample generated more carbon species during the reaction due to the small 8-ring size, while those amounts of carbon species were restricted in the hierarchical pore structure and plate-like morphology in SP18 and SP34 samples.
December, 2023 | DOI: 10.1016/j.micromeso.2023.112792
Materiales de Diseño para la Energía y Medioambiente
Plasticized, greaseproof chitin bioplastics with high transparency and biodegradability
Heredia-Guerrero, JA; Benitez, JJ; Porras-Vazquez, JM; Tedeschi, G; Morales, Y; Fernandez-Ortuno, D; Athanassiou, A; Guzman-Puyol, SFood Hydrocolloids, 145 (2023) 109072
Show abstract ▽
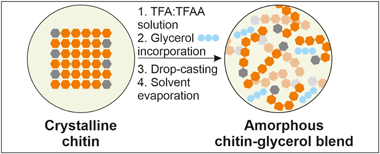
A mixture of trifluoroacetic acid:trifluoroacetic anhydride (TFA:TFAA) was used to dissolve chitin from shrimp shells. Free-standing films were prepared by blending the chitin solution and glycerol at different percentages, followed by drop-casting, and the complete evaporation of the solvents. After this process, the chitin matrix showed an amorphous molecular structure, as determined by X-ray diffraction. Optical, mechanical, thermal, and antioxidant properties were also thoroughly investigated. The incorporation of glycerol induced a plasticizing effect on the mechanical response of films and improved their transparency. In addition, hydrodynamic and barrier properties were determined by contact angle and water vapor/oxygen transmission rates, respectively, and revealed typical values of other polysaccharides. These bioplastics also presented an excellent greaseproof behavior with the highest degree of oil repellency as determined by the Kit test. Moreover, the overall migration was evaluated by using Tenax & REG; as a dry food simulant and levels were compliant with European regulations. Their antifungal properties were tested using Botrytis cinerea as a model. Biodegradability was also determined by measuring the biological oxygen demand in seawater. Degradation rates were high and similar to those of other fully-degradable materials.
December, 2023 | DOI: 10.1016/j.foodhyd.2023.109072
- ‹ previous
- 6 of 410
- next ›














