Scientific Papers in SCI
2024
2024
Tribología y Protección de Superficies
Synthesis and Characterization of Multilayered CrAlN/Al2O3 Tandem Coating Using HiPIMS for Solar Selective Applications at High Temperature
Sánchez-Pérez, M; Rojas, TR; Reyes, DF; Ferrer, FJ; Farchado, M; Morales, A; Escobar-Galindo, R; Sánchez-López, JCACS Applied Energy Materials, 7 (2024) 438-449
Show abstract ▽
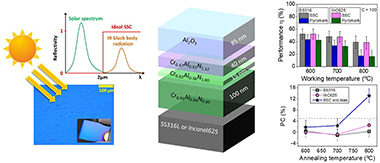
The effect of applying a negative bias during deposition of a previously designed multilayer solar selective absorber coating was studied on two types of substrates (316L stainless steel and Inconel 625). The solar selective coating is composed of different chromium aluminum nitride layers deposited using a combination of radiofrequency (RF), direct current (DC), and high-power impulse magnetron sputtering (HiPIMS) technologies. The chemical composition is varied to generate an infrared reflective/absorber layer (with low Al addition and N vacancies) and two CrAlN intermediate layers with medium and high aluminum content (Al/Cr = 0.6 and 1.2). A top aluminum oxide layer (Al2O3) is deposited as an antireflective layer. In this work, a simultaneous DC-pulsed bias (−100 V, 250 kHz) was applied to the substrates in order to increase the film density. The optical performance, thermal stability, and oxidation resistance was evaluated and compared with the performance obtained with similar unbiased coating and a commercial Pyromark paint reference at 600, 700, and 800 °C. The coating remained stable after 200 h of annealing at 600 °C, with solar absorptance (α) values of 93% and 92% for samples deposited on stainless steel and Inconel, respectively, and a thermal emittance ε25°C of 18%. The introduction of additional ion bombardment during film growth through bias assistance resulted in increased durability, thermal stability, and working temperature limits compared with unbiased coatings. The solar-to-mechanical energy conversion efficiency at 800 °C was found to be up to 2 times higher than Pyromark at C = 100 and comparable at C = 1000.
February, 2024 | DOI: 10.1021/acsaem.3c02310
Química de Superficies y Catálisis
Synthetic natural gas production using CO2-rich waste stream from hydrothermal carbonization of biomass: Effect of impurities on the catalytic activity
González-Arias, J; Torres-Sempere, G; Villora-Picó, JJ; Reina, TR; Odriozola, JAJournal of CO2 Utilization, 79 (2024) 102653
Show abstract ▽
The utilization of biomass and bio-waste, particularly through hydrothermal processes, has shown promise as a technology for converting these materials into valuable products. While most research has traditionally focused on the solid and liquid byproducts of these hydrothermal treatments, the gaseous phase has often been over-looked. This study specifically investigates the conversion of off-gases produced during hydrothermal carbonation (HTC) into synthetic natural gas, offering a readily marketable product with economic potential. Although the methanation of conventional flue gases has been extensively studied, dealing with non-standard off-gases from processes like HTC presents challenges due to the presence of minor impurities like CO and CH4. This novel research seeks to experimentally evaluate the methanation of HTC off-gases using nickel-based catalysts and analyze how these impurities affect the catalytic performance. The studied catalysts include nickel supported by ceria and alumina, as well as alumina supported nickel-cobalt systems. The results demonstrate that these catalysts exhibit high CO2 conversion and CH4 selectivity under ideal gas conditions. However, when real gas compositions with impurities are considered, CO2 conversion decreases at lower temperatures (ca. 20% lower conversion for real gas vs. ideal), probably due to side reactions such as CH4 cracking. This difference becomes less pronounced at higher temperatures. Nevertheless, the catalysts perform satisfactorily, especially at temperatures exceeding 350 degrees C. In conclusion, this study sheds light on the methanation of HTC off-gases and underscores the significance of understanding how impurities in real gases impact the process, providing potential directions for future research.
January, 2024 | DOI: 10.1016/j.jcou.2023.102653
Química de Superficies y Catálisis
Boosting Low-Temperature CO2 Hydrogenation over Ni-based Catalysts by Tuning Strong Metal-Support Interactions
Ye, RP; Ma, LX; Hong, XL; Reina, TR; Luo, WH; Kang, LQ; Feng, G; Zhang, RB; Fan, MH, Zhang, RGAngewandte Chemie-International Edition,
Show abstract ▽
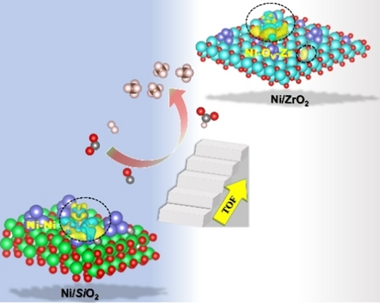
Rational design of low-cost and efficient transition-metal catalysts for low-temperature CO2 activation is significant and poses great challenges. Herein, a strategy via regulating the local electron density of active sites is developed to boost CO2 methanation that normally requires >350 °C for commercial Ni catalysts. An optimal Ni/ZrO2 catalyst affords an excellent low-temperature performance hitherto, with a CO2 conversion of 84.0 %, CH4 selectivity of 98.6 % even at 230 °C and GHSV of 12,000 mL g−1 h−1 for 106 h, reflecting one of the best CO2 methanation performance to date on Ni-based catalysts. Combined a series of in situ spectroscopic characterization studies reveal that re-constructing monoclinic-ZrO2 supported Ni species with abundant oxygen vacancies can facilitate CO2 activation, owing to the enhanced local electron density of Ni induced by the strong metal-support interactions. These findings might be of great aid for construction of robust catalysts with an enhanced performance for CO2 emission abatement and beyond.
January, 2024 | DOI: 10.1002/anie.202317669
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
CuO-TiO2 pilot-plant system performance for solar photocatalytic hydrogen production
Villachica-Llamosas, JG; Ruiz-Aguirre, A; Colón, G; Peral, J; Malato, SInternational Journal of Hydrogen Energy, 51 (2024) 1069-1077
Show abstract ▽
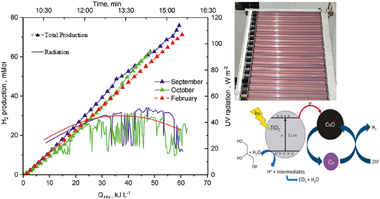
The main goal of the present study was to explore photocatalytic performance of the TiO2 -CuO mixture, for solar to hydrogen conversion at pilot plant scale under two different irradiation conditions (sunny and partly cloudy), focusing on high-temperature pretreat-ment of the catalyst mixture to try to improve TiO2 doping with copper. P25-TiO2 and commercial CuO were used with different amounts of Cu (2 wt% or 7 wt% Cu) calcined at 200-400 degrees C during several hours. Catalysts were tested at pilot plant scale using solar compound parabolic collectors, with glycerol as the sacrificial agent. The photocatalyst prepared after heating at 200 degrees C for 3 h and with 7 wt% Cu, resulted in higher hydrogen production than under the other heating conditions, and results were slightly better (5 -10%) than the reference values with the untreated catalysts. Photocatalytic efficiency was slightly lower at the higher calcination temperature (400 degrees C). CO2 production and formation of formate and glycolate clearly demonstrated glycerol photoreforming. The Cu from the calcined catalyst remaining on the solid was significantly less (2.5%) than on the non -calcined catalyst (4.2%), with an important fraction of lixiviated copper and copper deposition on the reactor walls. This is a critical drawback that must be considered for large-scale applications.
January, 2024 | DOI: 10.1016/j.ijhydene.2023.07.149
Química de Superficies y Catálisis
Switchable catalysis for methanol and synthetic natural gas synthesis from CO2: A techno-economic investigation
Merkouri, LP; Mathew, J; Jacob, J; Reina, TR; Duyar, MSJournal of CO2 Utilization, 79 (2024) 102652
Show abstract ▽
The oil and gas sector produces a considerable volume of greenhouse gas emissions, mainly generated from flaring and venting natural gas. Herein, a techno-economic analysis has been performed of a switchable catalytic process to convert the CH4 and CO2 in flared/vented natural gas into syngas or methanol. Specifically, it was shown that depending on greenhouse gas composition, dry methane reforming (DRM), reverse water-gas shift (RWGS), and CO2 methanation could be chosen to valorise emissions in an overall profitable and flexible operation scenario. The switchable process produced methanol and synthetic natural gas as its products, resulting in an annual income of €687m and annual operating expenses of €452m. The pre-tax profit was calculated at €234m, and at the end of the project, the net present value was calculated as €1.9b with a profitability index of 4.7€/€. The expected payback time of this process was ca. 4 years, and with a 35% internal rate of return (IRR). Most importantly, this process consumed 42.8m tonnes of CO2 annually. The sensitivity analysis revealed that variations in operation time, green hydrogen price, and products' prices significantly impacted the profitability of the process. Overall, this techno-economic analysis demonstrated that switchable catalysis in greenhouse gas utilisation processes is profitable, and thus it could play an important role in achieving net zero emissions.
January, 2024 | DOI: 10.1016/j.jcou.2023.102652
- ‹ previous
- 4 of 410
- next ›